
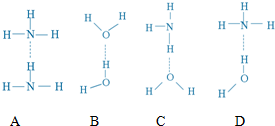

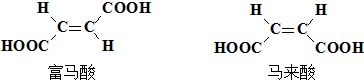
| ���� | H2 | N2H4 | H2NN��CH3��2 |
| �е�/�� | -252.8 | 113.5 | ��116 |
 ��
��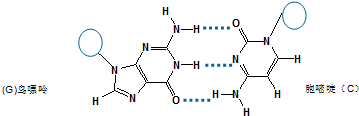 ��
��
 ��
��


| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��0.01mol/LNH4Al��SO4��2��Һ��0.02mol/LBa��OH��2��Һ�������ϣ�NH4++Al3++2SO42-+2Ba2++4OH-=2BaSO4��+Al��OH��3��+NH3?H2O |
| B������ͨ��ˮ�У�Cl2+H2O=Cl-+ClO-+2H+ |
| C��Ca��HCO3��2��Һ�еμӹ�����NaOH��Һ��Ca2++HCO3-+OH-=CaCO3��+H2O |
| D��������������Һ��ȥ�����������Ĥ��Al2O3+2OH-=2AlO2-+H2O |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��c=4g+10h |
| B��c-2b=2d+3f |
| C��2d+3f=3g+8h |
| D��a+c=d+f |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
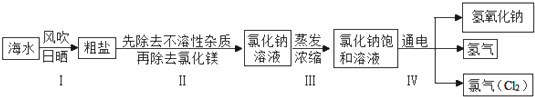

| ������ ������ | OH- | CO
| ||
| Ca2+ | | �� | ||
| Mg2+ | �� | |
| ʵ�鲽�� | ʵ������ | ʵ����� |
| ����һ��ȡһ�������Ȼ�����Һ���Թ��У�����������NaOH��Һ | ���������� | ˵��MgCl2 |
| �������������һ������Һ�м��������� | ������ɫ���� | ˵����Һ�к���CaCl2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
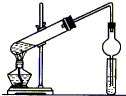 ��ij�������Ļ�����A������Է�������Ϊ104��̼����������Ϊ92.3%��
��ij�������Ļ�����A������Է�������Ϊ104��̼����������Ϊ92.3%��
| ϡ����KMnO4/OH- |

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A���٢ڢ� | B���ڢ� |
| C���ۢܢ� | D���ۢݢ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A���٢� | B���٢� | C���ۢ� | D���ڢ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��AgNO3 |
| B��HCl |
| C��NaOH |
| D��NaCl |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com