
����������Ҫ������Դ��������Ϊ�������õĹؼ��������ǵ�ǰ��ע���ȵ�֮һ��
�������������Դ����ȼ�ղ���Ϊ ��
��NaBH4��һ����Ҫ�Ĵ������壬����ˮ��Ӧ����NaBO3���ҷ�Ӧǰ��B�Ļ��ϼ۲��䣬�÷�Ӧ�Ļ�ѧ����ʽΪ����Ӧ����1molNaBH4ʱת�Ƶĵ�����ĿΪ��
�Ǵ���ɽ����л�������û�����ͱ�֮��Ŀ��淴Ӧ��ʵ������ͼ��⡣

 (g)
(g)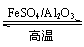


 (g)��3H2(g)
(g)��3H2(g)
��ij�¶��£�����������м��뻷���飬����ʼŨ��Ϊamol��L��1��ƽ��ʱ����Ũ��Ϊbmol��L��1���÷�Ӧ��ƽ�ⳣ��K��
��һ�������£���11ͼ��ʾװ�ÿ�ʵ���л���ĵ绯ѧ���⣨���������л����


�ٵ����е���ת�Ʒ���Ϊ ������A��D��ʾ��
������Ŀ�����ĵ缫��ӦʽΪ ��
�۸ô���װ�õĵ���Ч�ʦǣ� �����ǣ�����Ŀ��������ĵĵ�����/ת�Ƶĵ���������100%������������С�����1λ����
 ��У���˿��ֿ���ϵ�д�
��У���˿��ֿ���ϵ�д� �ƸԴ��ž�ϵ�д�
�ƸԴ��ž�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ԫ�������ɣ�±��Ԫ�ص��������ʴ��ϵ������εݼ�����
A���ǽ����� B��ԭ�Ӱ뾶
C�����ʵ������� D���⻯����ȶ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����йس�ȥ����(����������)�IJ����У�����ȷ����
A��FeCl2(FeCl3)���������������Ȼ�����
B����(����)������NaOH��Һ��Ȼ���Һ
C����ȩ(����)���������Ƶ���ʯ�ң�Ȼ������
D����������(����)�������Ҵ���Ũ���ᣬȻ�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
LiPF6������ӵ���й㷺Ӧ�õĵ���ʡ�ij������LiF��PCl5Ϊԭ�ϣ����·�Ӧ�Ʊ�LiPF6�����������£�
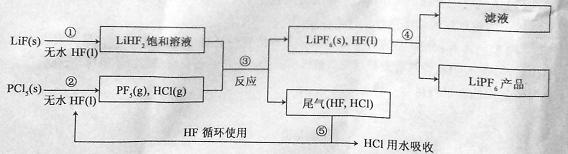
��֪��HCl�ķе��ǣ�85.0 �棬HF�ķе���19.5 �档
��1���ڢٲ���Ӧ����ˮHF�������� �� ����Ӧ�豸�����ò������ʵ�ԭ���� (�û�ѧ����ʽ��ʾ)����ˮHF�и�ʴ�ԺͶ��ԣ�������ȫ�ֲ���ʾ�������С�Ľ�HFմ��Ƥ���ϣ���������2%�� ��Һ��ϴ��
��2��������������ˮ�����½��У��ڢ۲���Ӧ��PCl5����ˮ�⣬�����Ϊ�����ᣬд��PCl5ˮ��Ļ�ѧ����ʽ�� ��
��3���ڢܲ�������õķ����� ���ڢݲ�����β����HF��HCl���õķ����� ��
��4��LiPF6��Ʒ��ͨ����������LiF��ȡ��Ʒwg�����Li�����ʵ���Ϊnmol�������Ʒ��LiPF6�����ʵ���Ϊ mol(�ú���w��n�Ĵ���ʽ��ʾ)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��֪C(s)��H2O(g)��CO(g)��H2(g) ��H��akJ��mol��1
2C(s)��O2(g)��2CO(g) ��H����220kJ��mol��1
H��H��O��O��O��H���ļ��ֱܷ�Ϊ436��496��462kJ��mol��1,��aΪ( )
A����332 B����118 C����350 D����130
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
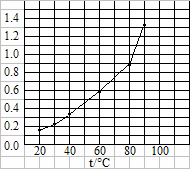
��������AgBrO3���ܽ�����¶ȱ仯������ͼ
��ʾ������˵���������
A�����������ܽ���Ƿ��ȹ���
B���¶�����ʱ�������ܽ��ٶȼӿ�
C��60��ʱ��������KspԼ����6��10-4
 D����������к��������������������ؽᾧ�����ᴿ
D����������к��������������������ؽᾧ�����ᴿ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����˵������ȷ����
A�������ԭˮ����ȵ��ˮ��������ܻ�����������
B��������ˮ(��NH4+��NH3)���û�ѧ��������绯ѧ����������
C��ij�ֹ�ѧ��⼼�����м��ߵ������ȣ��ɼ�����ϸ��(V=10��12L)�ڵ�����Ŀ����ӣ��ݴ˿�����ü�⼼���ܲ�����ϸ����Ũ��ԼΪ10��12��10��11mol ·L��1��Ŀ�����
D�������������Ӽ״��û��ȼ�ϵ���ֵ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�±���ÿ�����4��ѡ����������1��ѡ�������3��ѡ�����ڲ�ͬ�ķ��࣬�뽫������ѡ�����ż���ѡ���������±���
| ��� | ��ѡ�� | ����ѡ ����� | ��ѡ���� |
| (1) | A.S2�� B��I�� C��Fe��D��SO | ||
| (2) | A.HCl B��CO2 C��NH3 | ||
| (3) | A.����B����Һ C.������D���к� | ||
| (4) | A.KMnO4��B��Al2(SO4)3 C.KClO3�� D��K2HPO4 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
д�����е������ʵĵ��뷽��ʽ
(1)H2SO4_________________________________________________________________��
(2)H2CO3_________________________________________________________________��
(3)Ca(OH)2________________________________________________________________��
(4)NH3��H2O_______________________________________________________________��
(5)NaCl___________________________________________________________________��
(6)BaSO4_________________________________________________________________��
(7)NaHSO4________________________________________________________________��
(8)NaHCO3________________________________________________________________��
(9)NaHSO4(����)___________________________________________________________��
(10)CH3COOH_____________________________________________________________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com