

 ��ɢ˼ά�¿���ϵ�д�
��ɢ˼ά�¿���ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��15�֣�������Ԫ�����ڱ���һ���֣����ݸ�����Ԫ�ػش���������
��1��K��Ԫ�ط����� ��
��2��DԪ�ص���Χ�����Ų�ͼ�� �����仯�����У���������� ��
��3��CԪ�ص�ԭ�ӽṹʾ��ͼ�� ��
��4��A��L��B��L���ֱ����γ�ԭ�Ӹ�����Ϊ1��1�Ļ����A��L��1��1���Ļ������� ���ӣ���Ի�Ǽ��ԣ���B��L��1��1���Ļ�����ĵ���ʽ�� ��
��5��G��H��Lԭ�ӵĵ�һ�������ɸߵ��͵�˳���� ����Ԫ�ط��ţ���
��6��J���ʵ�һ�ְ�ɫͬ���칹��Ŀռ乹���� ��
��7��EԪ����Ԫ�����ڱ��е�λ���� �����仯�����У����У�2����3�۵�2�����ӣ���3�����ӱȽ��ȶ�����ԭ���� ��
��8��A��B�Ļ�������۵��A��L�Ļ�������۵�ߣ���ԭ���� ��
��9��A�ĵ�����L�ĵ�����B������������ˮ������Һ�����ȼ�յ�أ�д�����ֵ�صĸ����ĵ缫��Ӧʽ ��
��10��FԪ�ص������������MԪ�ص�����������ˮ���ﷴӦ�Ļ�ѧ����ʽ��
��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010��2011ѧ�����ʡ����һ�и߶���ѧ����ĩ���Ի�ѧ�Ծ� ���ͣ������
��15�֣�������Ԫ�����ڱ���һ���֣����ݸ�����Ԫ�ػش���������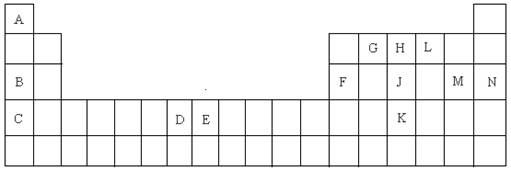
��1��K��Ԫ�ط����� ��
��2��DԪ�ص���Χ�����Ų�ͼ�� �����仯�����У���������� ��
��3��CԪ�ص�ԭ�ӽṹʾ��ͼ�� ��
��4��A��L��B��L���ֱ����γ�ԭ�Ӹ�����Ϊ1��1�Ļ����A��L��1��1���Ļ������� ���ӣ���Ի�Ǽ��ԣ���B��L��1��1���Ļ�����ĵ���ʽ�� ��
��5��G��H��Lԭ�ӵĵ�һ�������ɸߵ��͵�˳���� ����Ԫ�ط��ţ���
��6��J���ʵ�һ�ְ�ɫͬ���칹��Ŀռ乹���� ��
��7��EԪ����Ԫ�����ڱ��е�λ���� �����仯�����У����У�2����3�۵�2�����ӣ���3�����ӱȽ��ȶ�����ԭ���� ��
��8��A��B�Ļ�������۵��A��L�Ļ�������۵�ߣ���ԭ���� ��
��9��A�ĵ�����L�ĵ�����B������������ˮ������Һ�����ȼ�յ�أ�д�����ֵ�صĸ����ĵ缫��Ӧʽ ��
��10��FԪ�ص������������MԪ�ص�����������ˮ���ﷴӦ�Ļ�ѧ����ʽ��
��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010������ʡ�����߿���̾����壩�����ۺϻ�ѧ���� ���ͣ������
��15�֣�������Ԫ�����ڱ���ʾ��ͼ������բ�~���ڱ��е�λ�ã��û�ѧ����ش��������⣺

��1��Ԫ�آں͢ݵĵ��ʺͻ�����֮����һ�������´��ڷ�Ӧ�� ������
������ Ϊֱ���εķǼ��Է��ӡ�
Ϊֱ���εķǼ��Է��ӡ� �ĽṹʽΪ_________��
�ĽṹʽΪ_________��
��2��Ԫ�آ۵���̬�⻯��������������Ӧ��ˮ����֮����Է�Ӧ�����Σ�������ʵ�������е�һ����;Ϊ___________________________��
��3��Ԫ�آĵ��ʼ�����ǿ�ᷴӦ��������ǿ�Ӧ����Ԫ�آĵ��ʿ����ڸ��������»�ԭ�������������÷�Ӧ�Ļ�ѧ����ʽΪ___________________________��
��4���ɱ�������Ԫ�ص�ԭ�Ӱ�1��1��ɵij���Һ̬�������ϡ��Һ�ױ����ֽ⣬��ʹ�õĴ���Ϊ_________����һ�����ʵĻ�ѧʽ����
��5���ɱ���Ԫ���γɵij�������X��Y��Z��M��N�ɷ������·�Ӧ��

��X��Һ��Y��Һ��Ӧ�����ӷ���ʽΪ___________________________��
�� �ĵ��ʻ�ѧ����ʽΪ___________________________��
�ĵ��ʻ�ѧ����ʽΪ___________________________��
�۳����£�Ϊʹ ��Һ����
��Һ���� ���������������Ũ����ȣ�Ӧ����Һ�м���һ������Y��Һ��__________________��
���������������Ũ����ȣ�Ӧ����Һ�м���һ������Y��Һ��__________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012�����ʡ�߶���ѧ����ĩ���Ի�ѧ�Ծ� ���ͣ������
��15�֣�������Ԫ�����ڱ���һ���֣����ݸ�����Ԫ�ػش���������
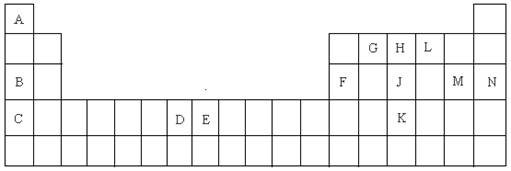
��1��K��Ԫ�ط����� ��
��2��DԪ�ص���Χ�����Ų�ͼ�� �����仯�����У���������� ��
��3��CԪ�ص�ԭ�ӽṹʾ��ͼ�� ��
��4��A��L��B��L���ֱ����γ�ԭ�Ӹ�����Ϊ1��1�Ļ����A��L��1��1���Ļ������� ���ӣ���Ի�Ǽ��ԣ���B��L��1��1���Ļ�����ĵ���ʽ�� ��
��5��G��H��Lԭ�ӵĵ�һ�������ɸߵ��͵�˳���� ����Ԫ�ط��ţ���
��6��J���ʵ�һ�ְ�ɫͬ���칹��Ŀռ乹���� ��
��7��EԪ����Ԫ�����ڱ��е�λ���� �����仯�����У����У�2����3�۵�2�����ӣ���3�����ӱȽ��ȶ�����ԭ���� ��
��8��A��B�Ļ�������۵��A��L�Ļ�������۵�ߣ���ԭ���� ��
��9��A�ĵ�����L�ĵ�����B������������ˮ������Һ�����ȼ�յ�أ�д�����ֵ�صĸ����ĵ缫��Ӧʽ ��
��10��FԪ�ص������������MԪ�ص�����������ˮ���ﷴӦ�Ļ�ѧ����ʽ��
��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
������Ԫ�����ڱ���һ���֣����ݸ�����Ԫ�ػش���������

��1��K��Ԫ�ط����� ��
��2��DԪ�ص���Χ�����Ų�ͼ�� �����仯�����У���������� ��
��3��CԪ�ص�ԭ�ӽṹʾ��ͼ�� ��
��4��A��L��B��L���ֱ����γ�ԭ�Ӹ�����Ϊ1��1�Ļ����A��L��1��1���Ļ������� ���ӣ���Ի�Ǽ��ԣ���B��L��1��1���Ļ�����ĵ���ʽ�� ��
��5��G��H��Lԭ�ӵĵ�һ�������ɸߵ��͵�˳���� ����Ԫ�ط��ţ���
��6��J���ʵ�һ�ְ�ɫͬ���칹��Ŀռ乹���� ��
��7��EԪ����Ԫ�����ڱ��е�λ���� �����仯�����У����У�2����3�۵�2�����ӣ���3�����ӱȽ��ȶ�����ԭ���� ��
��8��A��B�Ļ�������۵��A��L�Ļ�������۵�ߣ���ԭ���� ��
��9��A�ĵ�����L�ĵ�����B������������ˮ������Һ�����ȼ�յ�أ�д�����ֵ�صĸ����ĵ缫��Ӧʽ ��
��10��FԪ�ص������������MԪ�ص�����������ˮ���ﷴӦ�Ļ�ѧ����ʽ��
��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com