

| 3 |
| 2 |
| 3 |
| 2 |
| 1 |
| 2 |
| 1 |
| 8 |
| 6-2��2 |
| 2 |
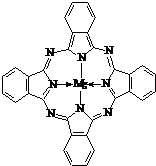 ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
��


 �ƸԹھ��ο���ϵ�д�
�ƸԹھ��ο���ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��H+��NO3-��Fe2+��Na+ |
| B��K+��Ba2+��OH-��SO42- |
| C��Fe2+��NO3-��I-��K+ |
| D��Cu2+��NH4+��Cl-��OH- |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��ԭ�Ӱ뾶��Z��R��W |
| B����̬�⻯���ȶ��ԣ�HW��H2R |
| C��XW4�����и�ԭ�Ӿ�����8���ӽṹ |
| D��Y��Z��R����Ԫ����ɵĻ�����ˮ��Һһ���Լ��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
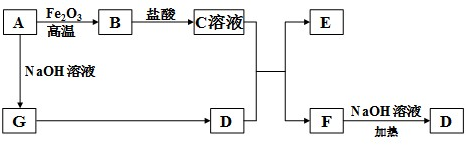
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| 15 |
| 16 |
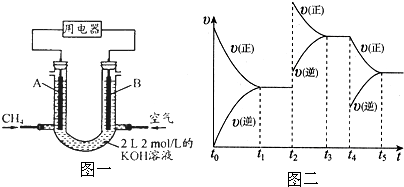
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 20.00mLһ�����ʵ���Ũ�ȵ�����X����һ��Ũ�ȵ�NaOH��ҺY�ζ����ζ���������ҺpH������NaOH��Һ������Ĺ�ϵ��ͼ��ʾ��������仯���Բ��ƣ�
20.00mLһ�����ʵ���Ũ�ȵ�����X����һ��Ũ�ȵ�NaOH��ҺY�ζ����ζ���������ҺpH������NaOH��Һ������Ĺ�ϵ��ͼ��ʾ��������仯���Բ��ƣ��鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ���÷�Ӧ��Cu2Cl2+C2H2+2NH3��Cu2C2����Ȳ��ͭ����ɫ��+2NH4Cl�ɼ�����Ȳ��
���÷�Ӧ��Cu2Cl2+C2H2+2NH3��Cu2C2����Ȳ��ͭ����ɫ��+2NH4Cl�ɼ�����Ȳ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��0.1 mol/L��������Һ����NH4Al��SO4��2 ��NH4Cl ��NH3?H2O ��CH3COONH4��c��NH4+���ɴ�С��˳���ǣ��ڣ��٣��ܣ��� |
| B��NaHCO3��Һ��Na2CO3��Һ���һ�����ڣ�c��Na+��+c��H+��=c��OH-��+c��HCO3-��+c��CO32-�� |
| C��25��ʱ��pH=11�İ�ˮ��pH=3������������ϣ�c��NH4+����c��SO42-����c��OH-����c��H+�� |
| D�������£���100mL 0.1mol/L NH4HSO4��Һ�еμ�0.1mol/L NaOH��Һ�����ԣ������Һ�и�����Ũ�ȴ�С��ϵ��c��Na+����c��NH4+����c��SO42-����c��OH-��=c��H+�� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com