
”¾ĢāÄæ”潫µČĪļÖŹµÄĮæA”¢B»ģŗĻÓŚ2LµÄĆܱÕČŻĘ÷ÖŠ·¢Éś·“Ó¦£ŗ3A(g)+B(g)xC(g)+D(g)”£¾4minŗ󣬲āµĆDµÄÅضČĪŖ0.4 mo1”¤L-1£¬CµÄĘ½¾ł·“Ó¦ĖŁĀŹĪŖ0.lmo1”¤L-1”¤min-1£¬c(A)£ŗc(B)=3£ŗ5”£ĻĀĮŠĖµ·Ø²»ÕżČ·µÄŹĒ
A.xµÄÖµŹĒ1B.4minÄ©£¬AµÄ×Ŗ»ÆĀŹĪŖ60%
C.ĘšŹ¼Ź±AµÄÅضČĪŖ2.4mol”¤L-1D.4minÄŚv(B)=0.1 mol”¤L-1”¤min-1
”¾“š°ø”æB
”¾½āĪö”æ

(a-1.2): (a-0.4) = 3:5
½āµĆa = 2.4
A. ![]() £¬x =1£¬¹ŹAÕżČ·£¬²»·ūŗĻĢāŅā£»
£¬x =1£¬¹ŹAÕżČ·£¬²»·ūŗĻĢāŅā£»
B. 4minÄ©£¬ AµÄ×Ŗ»ÆĀŹĪŖ![]() £¬¹ŹB“ķĪ󣬷ūŗĻĢāŅā£»
£¬¹ŹB“ķĪ󣬷ūŗĻĢāŅā£»
C. øł¾Ż·ÖĪöµĆ³öĘšŹ¼Ź±AµÄÅضČĪŖ2.4mol”¤L-1£¬¹ŹCÕżČ·£¬²»·ūŗĻĢāŅā£»
D. øł¾ŻB”¢CĻµŹżĻąµČĖŁĀŹĻąµČ£¬µĆ³ö4minÄŚv(B)=0.1 mol”¤L-1”¤min-1£¬¹ŹDÕżČ·£¬²»·ūŗĻĢāŅā£»
×ŪÉĻĖłŹöĖł£¬“š°øĪŖB”£


 ĘŚÄ©³å“Ģ100·Ö““ŠĀ½š¾ķĶźČ«ŹŌ¾ķĻµĮŠ“š°ø
ĘŚÄ©³å“Ģ100·Ö““ŠĀ½š¾ķĶźČ«ŹŌ¾ķĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æ»·Óė»·Ö®¼ä¹²ÓĆĮ½øö»ņ¶ąøöĢ¼Ō×ӵĶą»·ĶéĢž³ĘĪŖĒÅ»·ĶéĢž£¬ĘäÖŠ¶ž»·[1.1.0]¶”Ķé (![]() )ŹĒĘäÖŠŅ»ÖÖ”£ĻĀĮŠ¹ŲÓŚøĆ»ÆŗĻĪļµÄĖµ·ØÕżČ·µÄŹĒ
)ŹĒĘäÖŠŅ»ÖÖ”£ĻĀĮŠ¹ŲÓŚøĆ»ÆŗĻĪļµÄĖµ·ØÕżČ·µÄŹĒ
A. ÓėC3H4ŹĒĶ¬ĻµĪļ
B. Ņ»ĀČ“śĪļÖ»ÓŠŅ»ÖÖ
C. Óė»·¶”Ļ©»„ĪŖĶ¬·ÖŅģ¹¹Ģå
D. ĖłÓŠĢ¼Ō×ÓæÉÄܶ¼“¦ÓŚĶ¬Ņ»Ę½Ćę
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æĻĀĮŠĖµ·ØÖŠ£¬ÄÜĖµĆ÷»ÆŃ§Ę½ŗāŅ»¶ØĻņÕż·“Ó¦·½ĻņŅĘ¶ÆµÄŹĒ£Ø””””£©
A. N2O4(g)![]() 2NO2(g)£¬øıäijŅ»Ģõ¼žŗó£¬ĘųĢåŃÕÉ«¼ÓÉī
2NO2(g)£¬øıäijŅ»Ģõ¼žŗó£¬ĘųĢåŃÕÉ«¼ÓÉī
B. H2(g)+I2(g)![]() 2HI(g)£¬µ„Ī»Ź±¼äÄŚĻūŗÄH2ŗĶHIµÄĪļÖŹµÄĮæÖ®±Č“óÓŚ1:2
2HI(g)£¬µ„Ī»Ź±¼äÄŚĻūŗÄH2ŗĶHIµÄĪļÖŹµÄĮæÖ®±Č“óÓŚ1:2
C. N2(g)+3H2(g) ![]() 2NH3(g)£¬øıäijŅ»Ģõ¼žŗó£¬NH3µÄĢå»ż·ÖŹżŌö¼Ó
2NH3(g)£¬øıäijŅ»Ģõ¼žŗó£¬NH3µÄĢå»ż·ÖŹżŌö¼Ó
D. 2SO2(g) +O2(g)![]() 2SO3(g)£¬ŗćĪĀŗćŃ¹Ģõ¼žĻĀ£¬³äČėHe
2SO3(g)£¬ŗćĪĀŗćŃ¹Ģõ¼žĻĀ£¬³äČėHe
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æ·“Ó¦2C£«O2===2COµÄÄÜĮæ±ä»ÆČēĻĀĶ¼ĖłŹ¾”£ĻĀĮŠĖµ·ØÕżČ·µÄŹĒ(””””)

A”¢12 g C(s)ÓėŅ»¶ØĮæO2(g)·“Ӧɜ³É14 g CO(g)·Å³öµÄČČĮæĪŖ110.5 kJ
B”¢øĆ·“Ó¦µÄČČ»Æѧ·½³ĢŹ½ŹĒ2C(s)£«O2(g)===2CO(g)””¦¤H£½£221 kJ
C”¢2 mol C(s)Óė×ćĮæO2(g)·“Ӧɜ³ÉCO2(g)£¬·Å³öµÄČČĮæ“óÓŚ221 kJ
D”¢øĆ·“Ó¦µÄ·“Ó¦ČȵČÓŚCO·Ö×ÓÖŠ»Æѧ¼üŠĪ³ÉŹ±ĖłŹĶ·ÅµÄ×ÜÄÜĮæÓėO2·Ö×ÓÖŠ»Æѧ¼ü¶ĻĮŃŹ±ĖłĪüŹÕµÄ×ÜÄÜĮæµÄ²ī
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æ£ØNH4)2Cr2O7æÉÓĆ×÷ÓŠ»śŗĻ³É“߻ƼĮ”¢Ć½Č¾¼Į”¢ĻŌÓ°ŅŗµČ”£Ä³»ÆѧŠĖȤŠ”×é¶Ō(NH4)2Cr2O7µÄ²æ·ÖŠŌÖŹ¼°×é³É½ųŠŠĢ½¾æ”£ŅŃÖŖ£ŗCr2O72-(³ČÉ«£©+H2O2CrO42-£Ø»ĘÉ«£©+2H+”£Ēė»Ų“šĻĀĮŠĪŹĢā£ŗ
£Ø1£©ŌŚŹŌ¹ÜÖŠ¼ÓČėÉŁĮæ(NH4)2Cr2O7¹ĢĢ壬µĪ¼Ó×ćĮæÅØKOHČÜŅŗ£¬Õńµ“”¢Ī¢ČČ£¬¹Ū²ģµ½µÄÖ÷ŅŖĻÖĻóŹĒ___”£
£Ø2£©ĪŖĢ½¾æ(NH4)2Cr2O7(Ħ¶ūÖŹĮæĪŖ252g/mol)µÄ·Ö½ā²śĪļ£¬°“ČēĶ¼Į¬½ÓŗĆ×°ÖĆ£¬ŌŚAÖŠ¼ÓČė5.040gѳʷ½ųŠŠŹµŃ锣
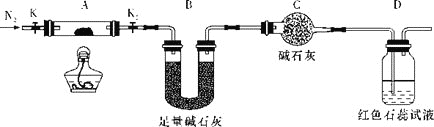
¢ŁĮ¬½ÓŗĆ×°ÖĆ£¬µćČ¼¾Ę¾«µĘÖ®Ē°£¬Šč½ųŠŠµÄ±ŲŅŖ²Ł×÷ŹĒ___”£
¢Ś·“Ó¦½įŹųŗó£¬ŅĄČ»ŅŖĶØŅ»¶ĪŹ±¼äµÄµŖĘųµÄŌŅņŹĒ___”£
¢Ū¼ÓČČAÖĮŗćÖŲ£¬¹Ū²ģµ½DÖŠČÜŅŗ²»±äÉ«£¬Ķ¬Ź±²āµĆAÖŠ²ŠĮōĪļĪŖCr2O3”¢BÖŠÖŹĮæµÄ±ä»ÆĪŖ1.44g£¬Š“³öÖŲøõĖįļ§¼ÓČČ·Ö½ā·“Ó¦µÄ»Æѧ·½³ĢŹ½£ŗ___”£
£Ø3£©ŹµŃéŹŅ³£ÓĆ¼×Č©·Ø²ā¶Øŗ¬(NH4)2Cr2O7µÄѳʷ֊µŖµÄÖŹĮæ·ÖŹż£ØŌÓÖŹ²»·¢Éś·“Ó¦£©£¬Ęä·“Ó¦ŌĄķĪŖ2Ba2++Cr2O72-+H2O=2BaCrO4”ż+2H+”¢4NH4++6HCHO=3H++6H2O+(CH2)6N4H+[µĪ¶ØŹ±£¬1mo1(CH2)6N4H+Óė1mo1H+Ļąµ±]£¬Č»ŗóÓĆNaOH±ź×¼ČÜŅŗµĪ¶Ø·“Ӧɜ³ÉµÄĖį”£
ŹµŃé²½Öč£ŗ³ĘȔѳʷ5.600g£¬Åä³É500mLČÜŅŗ£¬ŅĘČ”25.00mLѳʷČÜŅŗÓŚ250mL׶ŠĪĘæÖŠ£¬ÓĆĀČ»Æ±µČÜŅŗŹ¹Cr2O72-ĶźČ«³Įµķŗ󣬼ÓČė10mL20.00mol”¤L£1µÄÖŠŠŌ¼×Č©ČÜŅŗ£¬Ņ”ŌČ”¢¾²ÖĆ5minŗ󣬼ÓČė1”«2µĪ·ÓĢŖŹŌŅŗ£¬ÓĆ0.200mo1L-1NaOH±ź×¼ČÜŅŗµĪ¶ØÖĮÖÕµć”£ÖŲø“ÉĻŹö²Ł×÷3“Ī£¬×īÖÕµĪ¶ØÓĆČ„NaOH±ź×¼ČÜŅŗĢå»żµÄĘ½¾łÖµĪŖ20.00mL”£
¢ŁČōµĪ¶ØÖÕµćŹ±£¬ŃöŹÓ¶ĮŹż£¬Ōņ²ā¶Ø½į¹ū___(Ģī”°Ę«“ó”±”°Ę«Š””±»ņ”°ĪŽÓ°Ļģ”±£©”£
¢ŚµĪ¶Ø¼ĘĖćµĆøĆѳʷ֊NµÄÖŹĮæ·ÖŹżĪŖ___”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æĮ×¼°Ęä»ÆŗĻĪļŌŚ¹¤Å©ŅµÉś²śÖŠ¾ßÓŠÖŲŅŖÓĆĶ¾”£»Ų“šĻĀĮŠĪŹĢā£ŗ
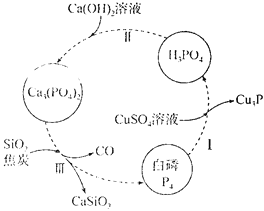
(1)ĻĀĶ¼ĖłŹ¾ĪŖĢį“æ°×Į×ѳʷ(ŗ¬¶čŠŌŌÓÖŹ)µÄ¹¤ŅÕĮ÷³Ģ”£¹ż³ĢIÖŠ£¬±»»¹ŌµÄŌŖĖŲŹĒ________(ĢīŌŖĖŲ·ūŗÅ)£¬¹ż³ĢIIIµÄ»Æѧ·½³ĢŹ½ĪŖ__________”£
(2)Į×Ėį·°ļ®/Ģ¼ø“ŗĻ²ÄĮĻ[Li3V2(PO4)3/C]ŹĒ³£ÓƵĵē¼«²ÄĮĻ£¬ĘäÖʱøĮ÷³ĢČēĻĀ£ŗ
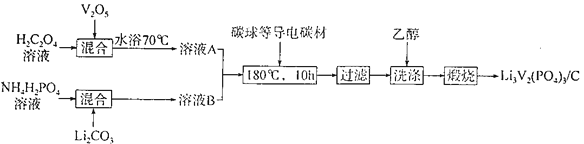
¢Łø“ŗĻ²ÄĮĻÖŠVµÄ»ÆŗĻ¼ŪĪŖ________£¬CµÄ×÷ÓĆŹĒ____________”£
¢ŚV2O5ÓėH2C2O4·“Ӧɜ³ÉV2(C2O4)3µÄ»Æѧ·½³ĢŹ½ĪŖ____________£»”°Ļ“µÓ”±Ź±ÓĆŅŅ“¼¶ų²»ÓĆĖ®µÄÄæµÄŹĒ________________”£
¢Ūļ®Ąė×Óµē³ŲŹĒŅ»ÖÖ¶ž“Īµē³Ų£¬ÓÖ³Ę”°Ņ”ŅĪ”±µē³Ų”£ČōÓĆŗĶLixC6ŗĶLi3V2(PO4)3/C×öµē¼«£¬·ÅµēŹ±µÄµē³Ų×Ü·“Ó¦ĪŖLixC6£«Li3£xV2(PO4)3= Li3V2(PO4)3+C6£¬Ōņµē³Ų³äµēŹ±Ńō¼«µÄµē¼«·“Ó¦Ź½ĪŖ___________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æĀČĘųŹĒŅ»ÖÖĒå½ą”¢øߊ§ŠĀÄÜŌ“£¬ Ņ²ŹĒÖŲŅŖµÄ»Æ¹¤ŌĮĻ”£
(1)ĶعżČČ»Æѧѻ·ŌŚ½ĻµĶĪĀ¶ČĻĀÓÉĮņ»ÆĒā·Ö½āÖʱøĒāĘųµÄ·“Ó¦ĻµĶ³ŌĄķĪŖ£ŗ
SO2(g)+I2(s)+2H2O(l)=2HI(aq)+H2SO4(aq) H1=-151kJmol-1
2HI(aq)=H2(g)+I2(s) H2=+110kJmol-1
H2S(g)+H2SO4(aq)=S(s)+SO2(g)+2H2O(l) H3=+61kJmol-1
(ČČ»ÆѧĮņµāŃ»·Įņ»ÆĒā·Ö½āĮŖ²śĒāĘų”¢Įņ»ĒĻµĶ³)
Ķعż¼ĘĖćæÉÖŖ£¬øĆĻµĶ³ÖĘĒāµÄČČ»Æѧ·½³ĢŹ½ĪŖ___________”£
(2)¹¤ŅµÉĻĄūÓĆCOŗĶH2ŗĻ³ÉĒå½ąÄÜŌ“CH3OH£¬Ęä·“Ó¦ĪŖCO(g)+2H2(g)CH3OH(g)¦¤H= -116 kJ”¤mol-1”£ČēĶ¼±ķŹ¾COµÄĘ½ŗā×Ŗ»ÆĀŹ(¦Į)ĖęĪĀ¶ČŗĶŃ¹Ēæ±ä»ÆµÄŹ¾ŅāĶ¼£ŗ
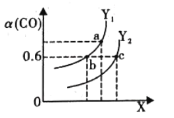
¢ŁX ±ķŹ¾µÄŹĒ______ (Ģī”°ĪĀ¶Č”±»ņ”°Ń¹Ēæ”±) £¬ĄķÓÉŹĒ_________£»Y1______Y2 (Ģī”°<”±”¢”° >”±»ņ”°=”±)
¢ŚŌŚ2LŗćČŻĆܱÕČŻĘ÷ÖŠ³äČė2 mol CO ŗĶ4 mol H2£¬Ņ»¶ØĢõ¼žĻĀ¾¹ż10 min “ļµ½Ę½ŗāדĢ¬c µć“¦”£ŌŚøĆĢõ¼žĻĀ£¬“ÓæŖŹ¼ÖĮ“ļµ½Ę½ŗāדĢ¬v(CH3OH) =______ molL-1min -1£¬Ę½ŗā³£ŹżK=________(Ģī×ī¼ņ·ÖŹż)”£Ę½ŗā³£ŹżKa”¢Kb”¢KcµÄ“󊔹ŲĻµŹĒ______
¢ŪĻĀĮŠ“ėŹ©¼ČÄÜŌö“ó·“Ó¦ĖŁĀŹÓÖÄÜĢįøß·“Ó¦Īļ×Ŗ»ÆĀŹµÄŹĒ______ (Ģī×ÖÄø)”£
A. Ź¹ÓĆ“ß»Æ¼Į B. ¼°Ź±·ÖĄėCH3OH C.ÉżøßĪĀ¶Č D.Ōö“óŃ¹Ēæ
(3) ŅŃÖŖČ¼ĮĻµē³ŲµÄ±ČÄÜ×īÓėµ„Ī»ÖŹĮæČ¼ĮĻĪļÖŹŹ§Č„µÄµē×ÓŹż³ÉÕż±Č”£ĄķĀŪÉĻH2”¢CH4”¢CH3OHµÄ¼īŠŌµē³ŲµÄ±ČÄÜĮæÓɓ󵽊”µÄĖ³ŠņĪŖ_________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”收»ÆѧÓėÉś»ī”·
£Ø1£©ŗĻĄķÉÅŹ³£¬±£³ÖÓŖŃų¾łŗā£¬ŹĒĒąÉŁÄźŃ§Éś½”浳ɳ¤µÄÖŲŅŖ±£Ö¤”£
¢Łµ°°×ÖŹŹĒČĖĢå±ŲŠčµÄÓŖŃųĪļÖŹ£¬ĖüŌŚČĖĢåÄŚ×īÖÕ·Ö½āĪŖ£Ø £©”£
A£®ĘĻĢŃĢĒ B£®°±»łĖį C£®Ö¬·¾Ėį
¢ŚĻÖÓŠĻĀĮŠĪåÖÖĪļÖŹ AŹ³ŃĪ BŹ³“× CĘ»¹ūÖ DĘĻĢŃĢĒ EĒąĆ¹ĖŲ£¬Ēė°“ĻĀĮŠŅŖĒóĢīæÕ(ĢīŠņŗÅ)”£
ø»ŗ¬Ī¬ÉśĖŲCµÄŹĒ £»æÉÖ±½Ó½ųČėŃŖŅŗ£¬²¹³äÄÜĮæµÄŹĒ £»Ó¦ÓĆ×ī¹ć·ŗµÄæ¹ÉśĖŲÖ®Ņ»µÄŹĒ £»¼“æÉ×÷ĪŖµ÷Ī¶¼Į£¬ÓÖæÉ×÷ĪŖ·ĄøƼĮµÄŹĒ £»Ź³ÓĆ¹ż¶ą»įŅżĘšŃŖŃ¹Éżøß”¢ÉöŌąŹÜĖšµÄ ”£
£Ø2£©²ÄĮĻŹĒČĖĄąÉś“ęŗĶ·¢Õ¹µÄĪļÖŹ»ł“”£¬ŗĻĄķŹ¹ÓĆ²ÄĮĻæÉŅŌøÄÉĘĪŅĆĒµÄÉś»ī”£
¢Ł¾ÓŹŅ×°ŠŽĖłŹ¹µÄČĖŌģ°å²Ä»įŹĶ·Å³öŅ»ÖÖ»Ó·¢ŠŌĪļÖŹ£¬³¤ĘŚ½Ó“„»įŅżĘš¹żĆōŠŌʤŃ×£¬ĆāŅß¹¦ÄÜŅģ³££¬øƻӷ¢ŠŌĪļÖŹŹĒ£Ø £©”£
A£®¾Ę¾« B£®ÕįĢĒ C£®¼×Č©
¢ŚŅ»°ćĒéæöĻĀ£¬ŗĻ½š±Č×é³ÉĖüµÄ³É·Ö½šŹōÓ²¶Č (Ģī“󔢊”)”£
¢Ū³£ÓĆĄ“×÷°ėµ¼ĢåµÄĪļÖŹŹĒ £ØĢī»ÆѧŹ½£©£»ÓĆ×÷Ź³Ę·±£ĻŹÄ¤µÄµÄøß·Ö×Ó²ÄĮĻµÄ½į¹¹¼ņŹ½ĪŖ ”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æ25”ꏱ£¬Ė®µÄµēĄė“ļµ½Ę½ŗā£ŗH2O![]() H£«£«OH££¬ĻĀĮŠŠšŹöÕżČ·µÄŹĒ£Ø £©
H£«£«OH££¬ĻĀĮŠŠšŹöÕżČ·µÄŹĒ£Ø £©
A.½«“æĖ®¼ÓČȵ½95”ꏱ£¬Kw±ä“ó£¬pH²»±ä£¬Ė®ČŌ³ŹÖŠŠŌ
B.Ļņ“æĖ®ÖŠ¼ÓČėĻ”°±Ė®£¬Ę½ŗāÄęĻņŅĘ¶Æ£¬c(OH-)Ōö“ó£¬Kw±äŠ”
C.Ļņ“æĖ®ÖŠ¼ÓČėÉŁĮæNa2CO3¹ĢĢ壬c(OH-)Ōö“ó£¬Kw²»±ä£¬Ó°ĻģĖ®µÄµēĄėĘ½ŗā
D.Ļņ“æĖ®ÖŠ¼ÓČėŃĪĖį£¬æÉŅÖÖĘĖ®µÄµēĄė£»¼ÓČė“×Ėį£¬æÉ“Ł½ųĖ®µÄµēĄė
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com