
�ȼҵ�е�ⱥ��ʳ��ˮ��ԭ��ʾ��ͼ����ͼ��ʾ��
��1����ҺA�������� ��
��2����ⱥ��ʳ��ˮ�����ӷ���ʽ�� ��
��3�����ʱ�����������������Һ��pH��2��3���û�ѧƽ���ƶ�ԭ��������������� ��
��4��������õ���ˮ�辫�ơ�ȥ����Ӱ���Ca2����Mg2����NH4����SO42��[c(SO42����c(Ca2��)]��������������(����ˮ����ҺA���Ե���)�� 
������a����ɳ�⣬�����е������� ��
�ڹ��̢��н�NH4��ת��ΪN2�����ӷ���ʽ��
��BaSO4���ܽ�ȱ�BaCO3��С�����̢��г�ȥ��������
��1��NaOH ��2�֣� ��2��2Cl����2H2O 2OH����H2����Cl2����2�֣�
2OH����H2����Cl2����2�֣�
��3��������ˮ��Ӧ��Cl2��H2O HCl��HClO������HCl��Ũ�ȿ�ʹƽ�������ƶ�������������ˮ�е��ܽ⣬�������������������2�֣�
HCl��HClO������HCl��Ũ�ȿ�ʹƽ�������ƶ�������������ˮ�е��ܽ⣬�������������������2�֣�
��4����Mg(OH)2 ��2�֣� ��2NH4����3Cl2��8OH����8H2O��6Cl����N2����2�֣�
��SO42����Ca2�� ��2�֣�
���������������1�����ʱ�ڵ缫�������£���Һ�е��������������������˶����������������������˶������Ե�ⱥ��ʳ��ˮʱNa����H���������˶����ŵ磬��H����Na���õ��ӣ�����H�����ȷŵ磬����ʽΪ2H��+ 2e��= H2��������H����ˮ������ģ���������H���IJ��Ϸŵ磬���ƻ���������Χˮ�ĵ���ƽ�⣬OH����Ũ�Ⱦ������������ҺA��������NaOH������Cl����OH����ʧ���ӣ�������������Cl�����ȷŵ磬����ʽΪ2Cl����2e����Cl2������˵�ⱥ��ʳ��ˮ�����ӷ���ʽΪ2Cl��+ 2H2O 2OH��+ H2��+ Cl2������2����������1�� ��3����������������������������������ˮ�����������з�ӦCl2��H2O
2OH��+ H2��+ Cl2������2����������1�� ��3����������������������������������ˮ�����������з�ӦCl2��H2O HCl��HClO������ƽ���ƶ�ԭ����֪���������Ũ�ȿ�ʹƽ�����淴Ӧ�����ƶ�������������ˮ�е��ܽ⣬�������������������4��������Һ�к���Mg2������������ҺA����NaOH��������Һ��pHʱ�������Mg(OH)2������������a�л�����Mg(OH)2������ˮ�к�����������������ǿ�����ԣ��ɽ�NH4+����ΪN2������������ԭ��Cl��������ʽΪ2NH4��+ 3Cl2 + 8OH��=8H2O + 6Cl��+ N2��������ת����ʵ�ʾ��dz����ܽ�ƽ����ƶ���һ��˵�����ܽ��С�ij���ת�����ܽ�ȸ�С�ij�������ʵ�֡�����BaSO4���ܽ�ȱ�BaCO3��С�����Լ���BaCO3����Һ�е�SO42���ͽ��Ba2+���ɸ����ܵ�BaSO4������ͬʱ��Һ�л�����Ca2+��CaCO3Ҳ�������������ʣ���˻�������CaCO3������
HCl��HClO������ƽ���ƶ�ԭ����֪���������Ũ�ȿ�ʹƽ�����淴Ӧ�����ƶ�������������ˮ�е��ܽ⣬�������������������4��������Һ�к���Mg2������������ҺA����NaOH��������Һ��pHʱ�������Mg(OH)2������������a�л�����Mg(OH)2������ˮ�к�����������������ǿ�����ԣ��ɽ�NH4+����ΪN2������������ԭ��Cl��������ʽΪ2NH4��+ 3Cl2 + 8OH��=8H2O + 6Cl��+ N2��������ת����ʵ�ʾ��dz����ܽ�ƽ����ƶ���һ��˵�����ܽ��С�ij���ת�����ܽ�ȸ�С�ij�������ʵ�֡�����BaSO4���ܽ�ȱ�BaCO3��С�����Լ���BaCO3����Һ�е�SO42���ͽ��Ba2+���ɸ����ܵ�BaSO4������ͬʱ��Һ�л�����Ca2+��CaCO3Ҳ�������������ʣ���˻�������CaCO3������
���㣺���鰢��٤��������������������֪ʶ��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
����������й�������Һ���ʱ�ı仯�����
(1)��ʯī���缫������ͼװ�õ��AlCl3��Һ���������������ݣ��������г������ɡ�������⣬��������������Һ�л��ɹ۲쵽�������� �����ʹ���������ӷ���ʽ�� ��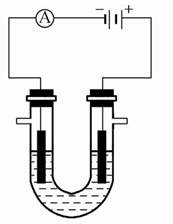
(2)����ʯī���缫���NaCl��Al2(SO4)3�Ļ����Һ�������Һ�ж��ߵ����ʵ���Ũ�ȷֱ�Ϊ3 mol��L-1��0.5 mol��L-1�������б�ʾ�����̵�������ȷ���� ��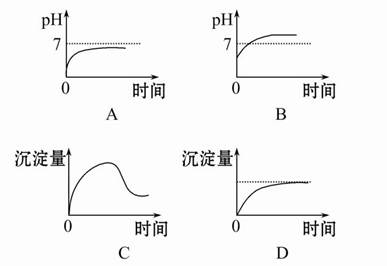
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
ij����С��ͬѧ����ͼװ�ý���ʵ��,�Իش���������:
(1)����ʼʱ����K��a����,��B���ĵ缫��ӦΪ ����
(2)����ʼʱ����K��b����,��B���ĵ缫��ӦΪ������������,�ܷ�Ӧ�����ӷ���ʽΪ�������������������й�����ʵ��,����˵����ȷ����(�����)��������
����Һ��Na+��A���ƶ����ڴ�A�����ݳ���������ʹʪ��KI������ֽ�������۷�Ӧһ��ʱ������������ɻָ������ǰ����ʵ�Ũ�ȡ�������״����B������2.24 L����,����Һ��ת��0.2 mol����
(3)��С��ͬѧģ�ҵ�������ӽ���Ĥ�����ռ�ķ���,������������ͼװ�õ���������Һ����ȡ������������������������ء�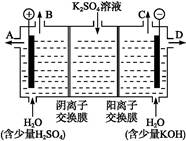
�ٸõ��۵�������ӦΪ����
��ʱͨ�������ӽ���Ĥ��������������������(����ڡ���С�ڡ����ڡ�)ͨ�������ӽ���Ĥ����������
��ͨ�翪ʼ��,����������ҺpH������,�����ԭ����������������������������������
�������Ƶõ�����������������������Һ���Ϊ����ȼ�ϵ��,���������ĵ缫��ӦΪ��������������������������������������������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
���������һ�����Ϳɳ���أ�����ͨ���ܵ����ȣ��õ�س�ʱ�䱣���ȶ��ķŵ��ѹ��������ص��ܷ�ӦΪ3Zn��2K2FeO4��8H2O 3Zn��OH��2��2Fe��OH��3��4KOH����Ҫ��ش��������⣺
3Zn��OH��2��2Fe��OH��3��4KOH����Ҫ��ش��������⣺
��1���ŵ�ʱ�� _______�������ʻ�ѧʽ����ͬ�������������ʱ��____________��������
��2���ŵ�ʱ����������Һ�ļ���__________ �����ǿ����������
��3�����ʱ��ÿת��3mol���ӣ����������ʵ����ʵ���Ϊ____________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��1����FeCl3��Һ��ʴӡˢ��·ͭ��Ļ�ѧ����ʽΪ�� �������÷�Ӧ��Ƴ���ͼ��ԭ��أ�����ͼ����ɱ�ע��
��2��ʵ��֤��������Ƴ�ԭ��صķ�Ӧͨ���Ƿ��ȷ�Ӧ���������з�Ӧ��
��C(s)+H2O(g)=CO(g)+H2(g) ��H>0��
��2H2(g)+O2(g)=2H2O(1) ��H<0 ��
��NaOH(aq)+HC1(aq)=NaC1(aq)+H2O(1) ��H<0 ��
������Ӧ������Ƴ�ԭ��ص��� ������ţ���������KOH��ҺΪ�������Һ��������ѡ��Ӧ��Ƶ�ԭ����为����ӦΪ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
ijʵ��С�����ݼ״�ȼ�յķ�Ӧԭ���������ͼ��ʾ��װ�á���֪�׳ص��ܷ�ӦʽΪ��2CH3OH+3O2+4KOH=2K2CO3+6H2O����ش�
��ͨ��O2�ĵ缫������ ��B�缫�������� ��
��ͨ��CH3OH�ĵ缫�ĵ缫��Ӧʽ�� ��A�缫�ĵ缫��ӦʽΪ ��
���ҳ����Ϊ1L����AgNO3����������µ��һ��ʱ�����Һ��PH��Ϊ1���������ʱ����ת�Ƶĵ�����ĿΪ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
���հ�ɽ��������������ɽ���ڣ�������½���ɽһ�������������׳ơ������͡�������������Ҫ�Ŀ���֮һ�������б���Ϊ����ʯ�����������ߣ�����ұ���̸ֵ���Ҫԭ�ϡ�����ʯ��Ҫ�ɷ��д�����Fe3O4��������FeCO3���̿�MnO2��MnCO3��ʯ��Mg3Si3O7(OH)4�ȡ���ҵ�Ͻ�����ʯ����������������Ĥ��ⷨ���¼�����ȡ�����̲��Ƶ���ɫ��Чˮ��������K2FeO4������ҵ�������£�
��1����ҵ��Ϊ���ϡ�����ȡЧ��һ���ȡ�Ĵ�ʩ�ǣ�����д���ַ�����
�� ��
��2��ʯ��ѧʽΪMg3Si3O7(OH)4Ҳ���Ա�ʾ����������ʽ�����������ʽΪ ��
��3����֪��ͬ����������������������������pH���±���
| ���� | Fe3+ | Al3+ | Fe2+ | Mn2+ | Mg2+ |
| ��ʼ������pH | 2.7 | 3.7 | 7.0 | 7.8 | 9.3 |
| ��ȫ������pH | 3.7 | 4.7 | 9.6 | 9.8 | 10.8 |

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
����������ԭ��Ӧ��2Ag+(aq)+Cu(s)=Cu2+(aq)+2Ag(s)��Ƶ�ԭ�������ͼ���ش��������⣺
��1���缫X�IJ����� ���������ҺY�� ��
��2�����缫Ϊ��ص� ����
��3���������������� �ƶ������ �����ҡ�����
��4�����·�е����Ǵ� ����缫������������ͬ���缫���� �缫��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��֪��1molH2��Ҫ����436kJ��������1molO2��Ҫ����496kJ�������γ�ˮ�����е�1molH��O�ܹ��ͷ�463kJ�����������������������ݼ��㷴Ӧ��
2H2(g)+O2(g)=2H2O(g) ����H = ��
��2����ͼ��ʾ�����γ�����ȼ�ϵ�ء�ͨ������ȼ�ϵ������ʽ(���������ҺΪH2SO4��Һʱ)�ͼ�ʽ[���������ҺΪNaOH(aq)��KOH(aq)ʱ]���֡��Իش��������⣺
����ʽ��صĵ缫��Ӧ������________________������______________������ܷ�Ӧ��______________���������ҺpH�ı仯________(��������С�����䡱)��
�ڼ�ʽ��صĵ缫��Ӧ������________________������______________������ܷ�Ӧ��______________���������ҺpH�ı仯________(��������С�����䡱)��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com