
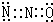 ��
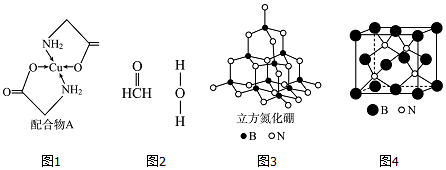
������ ��1��Cuԭ�Ӻ�����29�����ӣ����ݹ���ԭ����д���̬ԭ�Ӻ�������Ų�ʽ��
��2��ͬһ����Ԫ�أ�Ԫ�ص�һ����������ԭ��������������������ƣ�����IIA�塢��VA��Ԫ�ص�һ�����ܴ���������Ԫ�أ�Nԭ�Ӽ۲���ӶԸ�����4�����ݼ۲���ӶԻ��������ж�Nԭ���ӻ���ʽ��
��3��N2�ЦҼ��ͦм���Ŀ֮��Ϊ1��2���ȵ�����ṹ���ƣ����ݶ�����̼����ʽ��дN2O�ĵ���ʽ��
��4����ȩ������ƽ�������Σ�̼ԭ��λ���������ڲ����ṹ���Գƣ�����������C-H���н�С��120�㣻��ȩ�����е�Oԭ�Ӻ�ˮ�����е�Hԭ�����γ������
��5���þ�����Nԭ�Ӹ�����4��Bԭ�Ӹ���=8��$\frac{1}{8}$+6��$\frac{1}{2}$=4��������������Bԭ������ҵȾ��Nԭ�ӵ��������Ϊ��$\frac{1}{4}$��$\frac{1}{4}$��$\frac{1}{4}$����
��� �⣺��1��Cuԭ�Ӻ�����29�����ӣ����ݹ���ԭ����д���̬ԭ�Ӻ�������Ų�ʽΪ[Ar]3d104s1��
�ʴ�Ϊ��[Ar]3d104s1��
��2��ͬһ����Ԫ�أ�Ԫ�ص�һ����������ԭ��������������������ƣ�����IIA�塢��VA��Ԫ�ص�һ�����ܴ���������Ԫ�أ�����������Ԫ�ص�һ�����ܴ�С˳����N��O��C��Nԭ�Ӽ۲���ӶԸ�����4�����ݼ۲���ӶԻ��������ж�Nԭ���ӻ���ʽΪsp3��
�ʴ�Ϊ��N��O��C��sp3��
��3��N2�ЦҼ��ͦм���Ŀ֮��Ϊ1��2���ȵ�����ṹ���ƣ����ݶ�����̼����ʽ��дN2O�ĵ���ʽΪ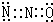 ���ʴ�Ϊ��1��2��
���ʴ�Ϊ��1��2�� ��
��
��4����ȩ��C��sp2�ӻ���C-H��C-H���н���������120�ȣ����������ʻ����ŶԵ��ӵ��ų⣬ʵ�ʼ���Ӧ����С��120�ȣ����Լ�ȩ������HCO�ļ��Ǵ���120�ȣ���ȩ�����е�Oԭ�Ӻ�ˮ�����е�Hԭ�����γ�������������ʾΪ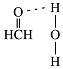 ��
��
�ʴ�Ϊ�����ڣ� ��
��
��5���þ�����Nԭ�Ӹ�����4��Bԭ�Ӹ���=8��$\frac{1}{8}$+6��$\frac{1}{2}$=4����B-N������Ϊ16����B-N����Bԭ�Ӹ���֮��Ϊ16��4=4��1��������������Bԭ������ҵȾ��Nԭ�ӵ��������Ϊ��$\frac{1}{4}$��$\frac{1}{4}$��$\frac{1}{4}$����
�ʴ�Ϊ��4��1����$\frac{1}{4}$��$\frac{1}{4}$��$\frac{1}{4}$����
���� ���⿼�����ʽṹ�����ʣ�Ϊ��Ƶ���㣬�漰�������㡢���ǡ�ԭ���ӻ���֪ʶ�㣬���ؿ���ѧ���Ի���֪ʶ��������ü������������ѵ��Ǿ������㼰ԭ���ӻ���ʽ�жϣ���Ŀ�Ѷ��еȣ�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����

| A�� | ͼ2 �з�Ӧ���������������� | |
| B�� | ͼ1 �������ҵķ�Ӧ����ʹ���˴��� | |
| C�� | ͼl ��������0��5 minʱ����v${\;}_{��{N}_{2}��}$=0.012mol/��L•min�� | |
| D�� | ͼ1 ���������ڷ�Ӧ��ƽ�ⳣ��Ϊ2.5 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
| ���� | ���� |
| ����ʢ��4.0gNa2O2���ձ��м���50mL����ˮ | ���ҷ�Ӧ��������������ʹ������ľ����ȼ������ȫ���ܽ�õ�����ɫ��Һa |
| ������Һa�е������η�̪ | ��Һ��죬10���Ӻ���Һ��ɫ���Ա�dz���Ժ���Һ��Ϊ��ɫ |
| ������Һ�м�������MnO2��ĩ | ���д������ݲ���������������Ҳ��ʹ������ľ����ȼ |
 �������ԣ�ʵ��֤ʵ����Һa��H2O2�Ĵ��ڣ�Ӧ��ͬλ��ʾ��ԭ�����Ա�
�������ԣ�ʵ��֤ʵ����Һa��H2O2�Ĵ��ڣ�Ӧ��ͬλ��ʾ��ԭ�����Ա��鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����

| A�� | ����ʽΪC14H18O6 | B�� | �����ǻ����Ȼ��ͱ��� | ||
| C�� | �ܷ���ȡ����Ӧ | D�� | ��ʹ���ˮ��Һ��ɫ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 78g���к��е�̼̼˫������ĿΪ3NA | |
| B�� | 16g��Cu2S��CuO��ɵĻ�����к��е���������Ϊ0.2NA | |
| C�� | ��1molH2��1molI2����һ�ܱ������г�ַ�Ӧ��ת�Ƶĵ�����Ϊ2NA | |
| D�� | 1mo1Fe��������Ũ���Ṳ�ȷ�Ӧ������SO2�ķ�����ΪNA |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
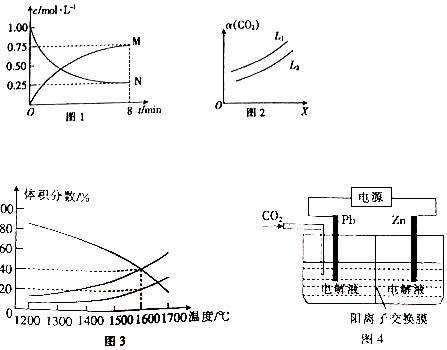
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
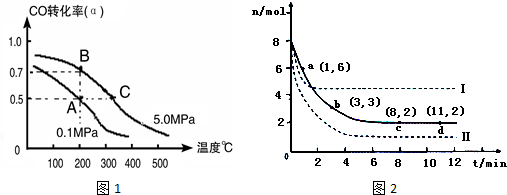
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����

| A�� | ���ڷ����� | B�� | �����к��������� | ||
| C�� | �ܱ����Ը��������Һ���� | D�� | �����ڱ� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com