
��I�����������ѳ�Ϊ��ǰ��δ����һ��ȫ���Կ��⡣Ϊ���Ŀǰȼ��ʹ�ù����еĻ�����Ⱦ���⣬��������ԴΣ�����е�ר���������̫���ܴ�ʹȼ��ѭ��ʹ�õĹ��룬��ͼ��ʾ��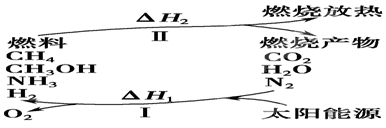
��ش��������⣺
(1)���̢������ת����ʽΪ________��ת��Ϊ________�ܡ�
(2)����ת�������У���H1�ͦ�H2�Ĺ�ϵ��________��
(3)����1 mol��ѧ��������������±���
| ���ۼ� | H��N | H��O | N��N | O��O |
| ����1 mol��ѧ����������/(kJ��mol��1) | 393 | 460 | 941 | 499 |
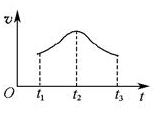 ���Խ���t1��t2��t2��t3���ʱ仯��ԭ��______________________________________________________��
���Խ���t1��t2��t2��t3���ʱ仯��ԭ��______________________________________________________�� ��I����1��̫�� 1�֣���ѧ (1��)�� ��2����H1������H2 (1��)
��3��2N2(g)+6H2O(g)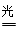 4NH3(g)+3O2(g) ��H��+1189kJ/mol
4NH3(g)+3O2(g) ��H��+1189kJ/mol (4��)
(4��)
����4��0.002md/(L��min) (2��)
��5��t1��t2˵��Mn2+��������t2��t3����Ӧ��Ũ�Ƚ��� (4��)
���������������I����1������ת��ʾ��ͼ��֪������I������̫���ܽ�������ˮ��CO2��ת��Ϊ�������������������״��ͼ���ȣ��������ת����̫����ת��Ϊ��ѧ�ܡ�
��2�����̢�����һ���������������������������״��ͼ����������ת��ΪCO2��ˮ�͵����ȣ����Ը��������غ��֪��H1������H2��
��3������ԭ���غ��֪��������ˮ��Ӧ���ɰ�����ͬʱ�����������ɣ���Ӧ�Ļ�ѧ����ʽΪ2N2+6H2O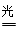 4NH3(g)+3O2�����ڷ�Ӧ�ȵ��ڶϼ����յ��������γɻ�ѧ�����ų��������IJ�ֵ����÷�Ӧ�ķ�Ӧ�ȡ�H��2��942kJ/mol��6��2��460kJ/mol��4��3��393kJ/mol��3��499kJ/mol����1189kJ/mol�����Ȼ�ѧ����ʽΪ2N2(g)+6H2O(g)
4NH3(g)+3O2�����ڷ�Ӧ�ȵ��ڶϼ����յ��������γɻ�ѧ�����ų��������IJ�ֵ����÷�Ӧ�ķ�Ӧ�ȡ�H��2��942kJ/mol��6��2��460kJ/mol��4��3��393kJ/mol��3��499kJ/mol����1189kJ/mol�����Ȼ�ѧ����ʽΪ2N2(g)+6H2O(g)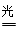 4NH3(g)+3O2(g) ��H��+1189kJ/mol��
4NH3(g)+3O2(g) ��H��+1189kJ/mol��
����4��5���Ӻ���Mn2+��Ũ��Ϊ0.004mol/L ������ݷ���ʽ��֪���IJ�������ʵ���Ũ����0.004mol/L�� ��0.01mol/L�����Է�Ӧ���ʦ�(H2C2O4)��0.01mol/L ��5min��0.002md/(L��min)��
��5������Ӱ�췴Ӧ���ʵ�����һ�����¶ȡ�Ũ�Ⱥʹ����ȣ���ϵ�¶Ȳ��䣬����Ӱ�췴Ӧ���ʵ����ؿ��Դ�Ũ�Ⱥʹ����ĽǶȷ�����t1��t2��Ӧ�������ߣ�˵��Mn2+���������ӿ��˷�Ӧ���ʣ���t2��t3��Ӧ�����ֽ��ͣ���˵����Ӧ��Ũ�Ƚ��͵��·�Ӧ���ʽ��͡�
���㣺���鷴Ӧ���������仯���Ȼ�ѧ����ʽ��д�Լ���Ӧ���ʼ������������Է�Ӧ���ʵ�Ӱ��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
(ÿ��1��,��7��)�ڻ�ѧ��Ӧ�У�ֻ�м�����������ƽ�������ߵö�ķ�Ӧ����ӷ�����ײʱ�ſ��ܷ�����ѧ��Ӧ����Щ���ӱ���Ϊ����ӡ�ʹ��ͨ���ӱ�ɻ���������ṩ������ȵ������л�ܣ��䵥λͨ����kJ��mol��1��ʾ��������۲���ͼ��Ȼ��ش����⡣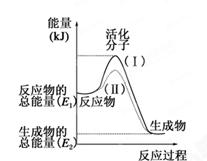
��1��ͼ����ʾ��Ӧ��________(����ȡ����ȡ�)��Ӧ��
��2����֪��1mol H��H����1mol I��I��1mol H��I���ֱ���Ҫ���յ�����Ϊ436kJ��151kJ��299kJ������1mol������1mol �ⷴӦ����HI��________(��ų��������ա�)________kJ���������ڻ�ѧ��Ӧ�����У��ǽ�______ת��Ϊ________��
��3�����з�Ӧ�У����ڷ��ȷ�Ӧ����________���������ȷ�Ӧ����________��
������ȼ�� ��ըҩ��ը ������кͷ�Ӧ �ܶ�����̼ͨ�����ȵ�̼
��ʳ�������������� ��Ba(OH)2��8H2O��NH4Cl��Ӧ ��������ϡ���ᷴӦ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��1���ϳɰ���ҵ������������Ȼ����ˮ��Ӧ�Ʊ�������Ҫ��ӦΪ��
CH4(g)+ 2H2O(g) 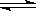 CO2(g)+4H2(g)
CO2(g)+4H2(g)
��Ӧ�����������仯��ͼ��ʾ��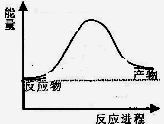
��÷�ӦΪ ��Ӧ������ȡ����ȡ���
����֪���ƻ�1mol��ѧ����Ҫ���յ��������±���ʾ��
| ��ѧ�� | C��H | O��H | C=O | H��H |
| ����������kJ/mol�� | a | b | c | d |
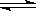 CO2(g)+4H2 (g)��Ӧ�� ��Ӧ���е�4sʱCO2��Ũ��Ϊ0.08mol/L���ٹ�һ��ʱ�䷴Ӧ��ƽ�⣬ƽ��ʱ������ѹǿ����ʼʱ��1.4����
CO2(g)+4H2 (g)��Ӧ�� ��Ӧ���е�4sʱCO2��Ũ��Ϊ0.08mol/L���ٹ�һ��ʱ�䷴Ӧ��ƽ�⣬ƽ��ʱ������ѹǿ����ʼʱ��1.4�����鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��14�֣����û�ѧ��Ӧԭ�����������������
��1����֪�� ��CO(g)+2H2(g)  CH3OH(g) ��Hl= ��91kJ��mol��l
CH3OH(g) ��Hl= ��91kJ��mol��l
��2CH3OH(g) CH3OCH3(g)+H2O(g) ��H2= ��24 kJ��mol��l ��CO(g) +H2O(g)
CH3OCH3(g)+H2O(g) ��H2= ��24 kJ��mol��l ��CO(g) +H2O(g)  CO2(g)+H2(g) ��H3= ��41 kJ��mol��l
CO2(g)+H2(g) ��H3= ��41 kJ��mol��l
��������Ӧ��ƽ�ⳣ������ΪK1��K2��K3 ��Ӧ 3CO(g) +3H2(g) CH3OCH3(g) +CO2(g) ��H= .
��Ӧ 3CO(g) +3H2(g) CH3OCH3(g) +CO2(g) ��H= .
��ѧƽ�ⳣ��K= ���ú�K1��K2��K3�Ĵ���ʽ��ʾ����
��2��һ�������£����������Ϊ1:2��CO��H2����ͨ�����һ�����ܱ������з�����Ӧ 3CO(g) +3H2(g) CH3OCH3(g) +CO2(g)��������˵����Ӧ�ﵽƽ��״̬�� ��
3CO(g) +3H2(g) CH3OCH3(g) +CO2(g)��������˵����Ӧ�ﵽƽ��״̬�� ��
a����ϵѹǿ���ֲ��� B����������ܶȱ��ֲ���
c�� CO��H2�����ʵ������ֲ��� d��CO�������ٶȵ���CO2����������
��3����������ˮ�õ���ˮ����25���£���x mol��L��l�İ�ˮ��y mol��L��1������������ϣ���Ӧ����Һ�����ԣ���c(NH4+)____c��Cl�������>������<������=�������ú�x��y�Ĵ���ʽ��ʾ����ˮ�ĵ���ƽ�ⳣ�� .
��4����ѧ�ҷ�����ʹNH3ֱ������ȼ�ϵ�صķ�������װ���ò������缫������������Һ�У�һ���缫ͨ���������һ�缫ͨ��NH3�����ط�ӦʽΪ��4NH3+3O2 = 2N2+6H2O���������ҺӦ�� ������ԡ��������ԡ��������ԡ�����
д�������ĵ缫��Ӧ����ʽ .
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��14�֣�
��.�ȼҵ�г������ӽ���Ĥ������Ƽ��ͼ1��ʾ����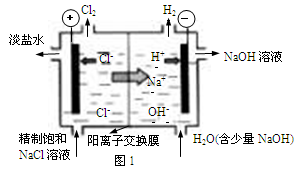
����1��д��ͼ1�������ĵ缫��Ӧʽ ��
����2����֪�����ӽ���Ĥֻ����������ͨ���������ӽ���Ĥֻ����������ͨ������ҵ������ͼ2װ�õ�ⱥ��Na2SO4��Һ������������NaOH��H2SO4�����װ������Ҫ��ȱ���� ��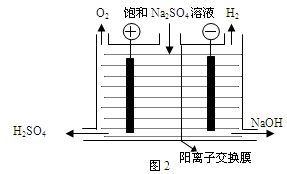
��.�����(MoS2)��һ����Ҫ�Ŀ��ͼ3�ǻ�����㱺��¯��ʾ��ͼ������1��2��3������¯���š�580��600��610�����Ǹ�¯����¶ȣ��棩��ͼ4�����˸�¯��������ϵ����ʵ����ٷֺ�����
��֪��MoS2��������1molMoO3�ķ�Ӧ�ȡ�H1=-1011KJ/mol��MoO2��������1molMoO3�ķ�Ӧ�ȡ�H2=-154KJ/mol���Իش�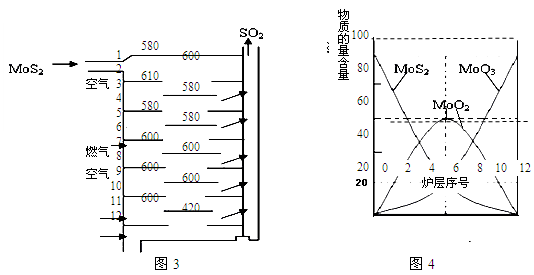
��1����֤����������ɵ�������SO2������SO3�ķ����� ��
��2������������ɵ�������ʹ�����ữ��KMnO4��Һ��ɫ���û�ѧ����ʽ��ʾ��ɫ��ԭ�� ��
��3����6¯����ڵĹ������ʷֱ���MoS2��MoO3��MoO2�������ǵ����ʵ���֮��Ϊ ��
��4��ͼ4�������м�¯�㣨4��6�����ܴ���һ�֡�����+���������+�����ķ�Ӧ����д���÷�Ӧ���Ȼ�ѧ��Ӧ����ʽ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��14�֣�2013��10��������̨��������ܵ��ش���ʧ���м������Ľ����ɹ�����ҩƷ���������ֺ���Ҫ�����������������������и�Ч���������̼�����������Ҫ�ɷ�H2O2��һ����ɫճ��Һ�壬��ش��������⣺
�Ż�����䳣��Һ̬�£�N2H4��Ϊȼ�ϣ�Һ̬H2O2Ϊ��ȼ������֪��
N2H4��1��+O2��g��=N2��g��+2H2O��g�� ��H="-" 534 kJ��mol��1 ��
H2O2��1��=H2O��1��+1/2O2��g�� ��H="-" 98��64 kJ��mol��1 ��
H2O��1��=H2O��g�� ��H=+44kJ��mol��l ��
��ӦN2H4��1��+2H2O2��1��=N2��g��+4H2O��g���ġ�H= ��
�ƾݱ����������⻯����NaBH4(BԪ�صĻ��ϼ�Ϊ��3��)��H2O2�� ԭ�ϵ�ȼ �ϵ�أ��������ϲ���Pt/C���������ϲ���MnO2���������վ�ͨ�����ǵ磬�乤��ԭ����ͼ��ʾ��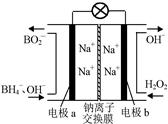
��õ�ص�������Ӧ____ ___
��H2O2��һ�ֲ��ȶ��ֽ�����ʡ���ͼ��H2O2��û�д���ʱ��Ӧ�����������仯ͼ������ͼ�ϻ���ʹ�ô����ӿ�ֽ�����ʱ���������ͼ ��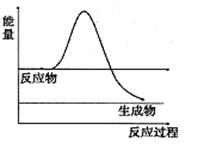
��ij��ѧ��ȤС�����ⶨH2O2�ķֽ����ʣ�ȡ��Һ0.5L���з����������������ʾ��
| t��S�� | 0 | 2 | 4 | 6 | 8 | 10 |
| n(H2O2) (moL) | 0.8 | 0.7 | 0.62 | 0.55 | 0.27 | 0.03 |

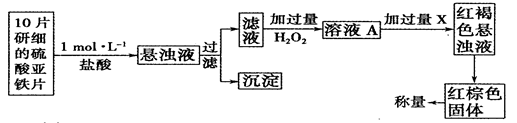
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��֪�� C(s)��O2(g)��CO2(g) ��H����393��5 kJ/mol
2CO(g)��O2(g)��2CO2(g) ��H����566 kJ/mol
TiO2(s)��2Cl2(g)��TiCl4(s)��O2(g) ��H��+141 kJ/mol
��TiO2(s)��2Cl2(g)��2C(s)��TiCl4(s)��2CO(g) ��H�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ο�����ͼ������֪E1��134 kJ��mol��1��E2��368 kJ��mol��1������Ҫ��ش����⣺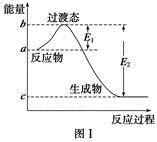
(1)ͼ����1 mol NO2(g)��1 mol CO(g)��Ӧ����CO2��NO�����е������仯ʾ��ͼ�����ڷ�Ӧ��ϵ�м����������Ӧ��������E1�ı仯�� (���������С�����䡱����ͬ)����H�ı仯�� ����д��NO2��CO��Ӧ���Ȼ�ѧ����ʽ�� ��
(2)�״����ӽ���Ĥȼ�ϵ���н��״�����ת��Ϊ���������ַ�Ӧ���Ȼ�ѧ����ʽ���£�
��CH3OH(g)��H2O(g)===CO2(g)��3H2(g) ��H����49.0 kJ��mol��1
��CH3OH(g)��O2(g)===CO2(g)��2H2(g) ��H����192.9 kJ��mol��1
��֪��H2O(g)===H2O(l) ��H����44 kJ��mol��1����״�����ȼ��ΪҺ̬ˮ���Ȼ�ѧ����ʽΪ ��
(3)�����ʾ�Dz��ֻ�ѧ���ļ��ܲ�����
| ��ѧ�� | P��P | P��O | O===O | P===O |
| ����/kJ��mol��1 | a | b | c | x |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
���Ȼ���(TiCl4)����ȡ���캽�չ�ҵ���ϡ����ѺϽ����Ҫԭ�ϡ���������(��Ҫ�ɷ���FeTiO3)�Ʊ�TiCl4�Ȳ�Ʒ��һ�ֹ�������ʾ��ͼ���£�
(1)�����м�����м������Һ����ɫ����ʱ��Һ�Գ�ǿ���ԡ��ù����������·�Ӧ������
Fe��2Fe3��=3Fe2��
2TiO2��(��ɫ)��Fe��4H��=2Ti3��(��ɫ)��Fe2����2H2O
Ti3��(��ɫ)��Fe3����H2O=TiO2��(��ɫ)��Fe2����2H��
������������� ��
(2)�ڢڡ��۹��չ�������Ҫ�����������γ�TiO2��nH2O�ܽ������ܽ��ķ�ɢ�ʿ���ֱ����С�� ��Χ��
(3)���Ѣ����ƵõĹ���TiO2��nH2O������ϴ��ȥ���е����ʣ������Ƶ��Ѱۡ���֪25 ��ʱ��Ksp[Fe(OH)3]��2.79��10��39�����¶��·�ӦFe(OH)3��3H�� Fe3����3H2O��ƽ�ⳣ��K�� ��
Fe3����3H2O��ƽ�ⳣ��K�� ��
(4)��֪��TiO2(s)��2Cl2(g)=TiCl4(l)��O2(g) ��H����140 kJ��mol��1
2C(s)��O2(g)=2CO(g)����H����221 kJ��mol��1
д������TiO2�ͽ�̿��������Ӧ����Һ̬TiCl4��CO������Ȼ�ѧ����ʽ�� ��
(5)�������վ��гɱ��͡����õ�Ʒλ����Ϊԭ�ϵ��ŵ㡣������ɫ��ѧ����ù��������д��ڵIJ���֮���� (ֻҪ��д��һ��)��
(6)�����±���Ϣ��Ҫ���ƺ�����SiCl4���ʵ�TiCl4���ɲ��� ������
| | TiCl4 | SiCl4 |
| �۵�/�� | ��25.0 | ��68.6 |
| �е�/�� | 136.4 | 57.6 |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com