
������й���ļ������Σ���������������ظ�
��Ԫ��֮Ϊ������NaCl����ṹ����ͼ��ʾ��
��1��������ÿ��Na+ͬʱ������______��Cl-��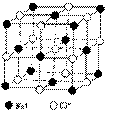
ÿ��Cl-ͬʱ������_______��Na+��
��2����������ÿ��Cl-��Χ������ӽ��Ҿ������
��Cl-����________����
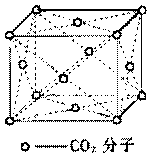
��3���ڸɱ�������ÿ��CO2������Χ���ڵ� CO2������___________���� �ھ����н�ȡһ����С�������Σ�ʹ�����ε��ĸ����㲿�䵽CO2���ӵ����ģ������������������___________��C02���ӡ����У�Cԭ�ӵ��ӻ�����Ϊ ��C02������̼��֮���Цм�����SiO2�й���֮���ަм���ԭ���� ��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ʡ���������ѧУ2011��2012ѧ��߶���ѧ����ĩ���Ի�ѧ���� ���ͣ�022
(1)�۵�е�HF________HI��ԭ��________��
(2)���ֺ�����HClO��HClO3��H2SO3��HClO4��������ǿ��������Ϊ________��
(3)����4�������۵�е��ɸߵ�������Ϊ________(�����)��
�ٽ��ʯ(C��C)����(Ge��Ge)
�۾����(Si��Si)�ܽ��ɰ(Si��C)
(4)������й���ļ������Σ���������������ظ���Ԫ��֮Ϊ������NaCl����ṹ����ͼ��ʾ��������ÿ��Na��ͬʱ������________��Cl��������ЩCl�����ɵļ��ι���Ϊ________��������ÿ��Cl����Χ������ӽ��Ҿ�����ȵ�Cl������________����

(5)ijԪ�صļ���̬ԭ�ӵĵ����Ų�ʽΪ1s22s22p63s23p34s1�����Ԫ�ػ�̬ԭ�ӵĵ����Ų�ʽΪ________��������������Ӧ��ˮ����Ļ�ѧʽ��________��
(6)ijԪ��ԭ�ӵļ۵��ӹ���Ϊ3d104S2�������ڵ�________���ڣ���________�壬________��Ԫ�أ�Ԫ�ط�����________����ԭ�Ӻ�����________���ܼ��������Ƶ���״��________�֣�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ�꼪��ʡ���������ѧУ�߶���ѧ����ĩ���Ի�ѧ�Ծ����������� ���ͣ������
��1�����۵�е�HF HI��ԭ��
��2�������ֺ�����HClO��HClO3��H2SO3��HClO4��������ǿ��������Ϊ
��3��������4�������۵�е��ɸߵ�������Ϊ________ ������ţ�
�ٽ��ʯ��C��C�����ࣨGe��Ge��
�۾���裨Si��Si���ܽ��ɰ��Si��C��
��4��������й���ļ������Σ���������������ظ���Ԫ��֮Ϊ������NaCl����ṹ��ͼ��ʾ��
������ÿ��Na+ͬʱ������______��Cl-������Щ Cl-���ɵļ��ι���Ϊ ��������ÿ��Cl-��Χ������ӽ��Ҿ�����ȵ�Cl-����________����
��5��ijԪ�صļ���̬ԭ�ӵĵ����Ų�ʽΪ1s 2s
2s 2p
2p 3s
3s 3p
3p 4s
4s �����Ԫ�ػ�̬ԭ�ӵĵ����Ų�ʽΪ ��������������Ӧ��ˮ����Ļ�ѧʽ��
�����Ԫ�ػ�̬ԭ�ӵĵ����Ų�ʽΪ ��������������Ӧ��ˮ����Ļ�ѧʽ��
��6��ijԪ��ԭ�ӵļ۵��ӹ���Ϊ3d104S2�������ڵ� ���ڣ��� �壬 ��Ԫ�أ�Ԫ�ط����� ����ԭ�Ӻ����� ���ܼ��������Ƶ���״�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013�켪��ʡ�߶���ѧ����ĩ���Ի�ѧ�Ծ��������棩 ���ͣ������
��1�����۵�е�HF HI��ԭ��
��2�������ֺ�����HClO��HClO3��H2SO3��HClO4��������ǿ��������Ϊ
��3��������4�������۵�е��ɸߵ�������Ϊ________ ������ţ�
�ٽ��ʯ��C��C�����ࣨGe��Ge��
�۾���裨Si��Si���ܽ��ɰ��Si��C��
��4��������й���ļ������Σ���������������ظ���Ԫ��֮Ϊ������NaCl����ṹ��ͼ��ʾ��

������ÿ��Na+ͬʱ������______��Cl-������Щ Cl-���ɵļ��ι���Ϊ ��������ÿ��Cl-��Χ������ӽ��Ҿ����� �ȵ�Cl-����________����
��5��ijԪ�صļ���̬ԭ�ӵĵ����Ų�ʽΪ1s 2s
2s 2p
2p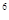 3s
3s 3p
3p 4s
4s �����Ԫ�ػ�̬ԭ�ӵĵ����Ų�ʽΪ
��������������Ӧ��ˮ����Ļ�ѧʽ��
�����Ԫ�ػ�̬ԭ�ӵĵ����Ų�ʽΪ
��������������Ӧ��ˮ����Ļ�ѧʽ��
��6��ijԪ��ԭ�ӵļ۵��ӹ���Ϊ3d104S2�������ڵ� ���ڣ��� �壬 ��Ԫ�أ�Ԫ�ط����� ����ԭ�Ӻ����� ���ܼ��������Ƶ���״�� ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com