
 4NO+6H2O���ʴ�Ϊ��4NH3+5O2
4NO+6H2O���ʴ�Ϊ��4NH3+5O2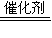 4NO+6H2O������д2NO+O2=2NO2Ҳ���ԣ���
4NO+6H2O������д2NO+O2=2NO2Ҳ���ԣ���

 Сѧ�̲�ȫ��ϵ�д�
Сѧ�̲�ȫ��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
(1)��С������к͵ζ�ʵ�飬ʵ�������ṩ�����漸����������ʽ�ζ��ܡ���ʽ�ζ��ܡ���Ͳ��������ƽ����ͨ©�������������ձ�������Ϊ��ȱ�ٵIJ���������__________��
(2)��С�����������ͼ��ʾ��һ��ʵ��װ�ã���̽����װ�õĶ���ԡ�

������ͬѧ��Ϊ��װ�ÿ���������ȡ���ռ�SO2���壬���Т���ʢ��NaOH��Һ����С��ͬѧ�������ۺ���Ϊ��װ�ô��ڰ�ȫ��������Ҫ������и�װ���뽫��װ���װ��ͼ���ڷ����ڡ�
����ǿͬѧ��Ϊ�����ø�װ�ÿ�����ȡ���������ռ����������Т���ʢ��Ũ���ᡣ����Ϊ�Ƿ���ȷ������ȷ��������������ȷ������Ľ����___________________��
(3)��С�������һ�һ��պ�ͭ���·��ϵĹ����������飬����Ա��ͬѧ��չʾ��������������������ͬѧ������������⣺
����1��

����2��

������Ϊ����___________________�����ϵ�ǰ��ɫ���������
��д������1�в����Ӧ�����ӷ���ʽ__________________��
�ۼ���Աָ���������Ƿ���1���Ƿ���2����Ӧ�����Թ�������м�����ճ�ȥ��������Ƴ�ȥ��м�IJ�������______________________________________________________��
��Ϊ�����Ӿ���Ч�棬�ù��������һ������dz��ɫ��Һ��ͨ��__________________����ȴ�ᾧ�����ˡ�ϴ�ӡ���Ȼ����õ�һ���׳ơ��̷����Ĺ�ҵ��Ʒ��
�ݱ���ͬѧ����ѯ���ϡ��о������ۣ�������˷���3�����ѷ���2�С���������ϡ���ᡢͨ����������ȡ���Ϊ����������ϡ�����H2O
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
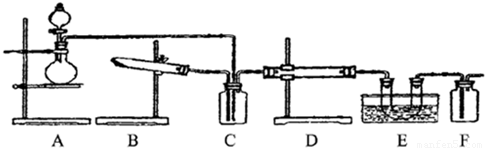
��15�֣�ij��ѧ��ȤС����ʵ����̽�������й����ʣ��������ͼ��ʾʵ�飬A�������巢��װ�ã�A�����õ�ʵ��ҩƷ������������ѡȡ��
�� Na2CO3 �� NaOH �� Ca(OH)2 �� NaHCO3 �� NH4Cl
����ͼ���Ӻø��������ֽ�C���IJ�˿�����������ȣ��ٽ�A������������ͨ��Bװ��Ƭ�̺�ȥC���ƾ��ơ�����ʵ����������£���˿�������ֺ���״̬��D�е�ͭƬ�����ܽ⣬�ش��������⣺
��1��A�з����Ļ�ѧ��Ӧ����ʽΪ________����ȡ�������õ��IJ���������Ҫ��_______�֣�����������װ�ã���
��2��Bװ�õ�����Ϊ____________________________��
��3��D��ͭƬ������Ӧ�����ӷ���ʽΪ___________________________��Ϊ��ʹCuƬ�ܽ�����ʼӿ죬����D������Һ�м����������������е�___________�������и�����ţ�
A��Na2CO3 B��AgNO3 C��H2SO4 D��FeSO4
��4��ͼE�г���ͨ������������Ϊ____________________��
��5��������Ϊ������Ƶ�����ʵ��װ�û��������ԵIJ��㣬���䲻��֮���Լ�Ӧ��θĽ���̸̸��������_______________________________________________________��
��ͨ������˵�������ײ�ǵ�����ײ���Ƿǵ�����ײ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ʡ��������У2010��ڶ���ģ�⿼�ԣ����ۣ���ѧ���� ���ͣ�ʵ����
��15�֣�ij��ѧ��ȤС����ʵ����̽�������й����ʣ��������ͼ��ʾʵ�飬A�������巢��װ�ã�A�����õ�ʵ��ҩƷ������������ѡȡ��
�� Na2CO3�� NaOH �� Ca(OH)2�� NaHCO3�� NH4Cl
����ͼ���Ӻø��������ֽ�C���IJ�˿�����������ȣ��ٽ�A������������ͨ��Bװ��Ƭ�̺�ȥC���ƾ��ơ�����ʵ����������£���˿�������ֺ���״̬��D�е�ͭƬ�����ܽ⣬�ش��������⣺
��1��A�з����Ļ�ѧ��Ӧ����ʽΪ________����ȡ�������õ��IJ���������Ҫ��_______�֣�����������װ�ã���
��2��Bװ�õ�����Ϊ____________________________��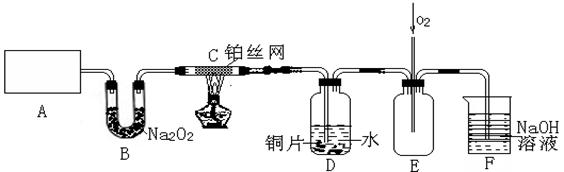 ��3��D��ͭƬ������Ӧ�����ӷ���ʽΪ___________________________��Ϊ��ʹCuƬ�ܽ�����ʼӿ죬����D������Һ�м����������������е�___________�������и�����ţ�
��3��D��ͭƬ������Ӧ�����ӷ���ʽΪ___________________________��Ϊ��ʹCuƬ�ܽ�����ʼӿ죬����D������Һ�м����������������е�___________�������и�����ţ�
| A��Na2CO3 | B��AgNO3 | C��H2SO4 | D��FeSO4 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013-2014ѧ�꽭��ʡ�Ž�����У�����ڶ���������ѧ�Ծ��������棩 ���ͣ�ʵ����
ij��ѧ��ȤС����ʵ����̽�������й����ʣ��������ͼ��ʾʵ�飬A�������巢��װ�ã�A�����õ�ʵ��ҩƷ������������ѡȡ��
��̼����?? ��̼������?? ��̼�����?? ���Ȼ��?? ����ʯ��?? ����������
����ͼ���Ӻø��������ֽ�C���IJ�˿�����������ȣ��ٽ�A������������ͨ��Bװ��Ƭ�̺�ȥC���ƾ��ƣ�����ʵ����������£���˿�������ֺ���״̬��D�е�ͭƬ�����ܽ⣬�ش��������⣺
��1����A����ȡ����ʱֻ����һ��ҩƷ�����ҩƷ��??? ����������ţ�
��2��Bװ�õ�����Ϊ??????????????
��3��D��ͭƬ������Ӧ�����ӷ���ʽΪ??????????? ��Ϊ��ʹCuƬ�ܽ�����ʼӿ죬����D������Һ�м����������������е�?????? �������и�����ţ�
A��Na2CO3 ???? B��AgNO3 ?????? C��H2SO4???????? D��FeSO4
��4��ͼE�г���ͨ������������Ϊ??????????? ��
��5��������Ϊ������Ƶ�����ʵ��װ�û��������ԵIJ��㣬���䲻��֮���Լ�Ӧ��θĽ���̸̸��������????????? ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ʡ��������У2010��ڶ���ģ�⿼�ԣ����ۣ���ѧ���� ���ͣ�ʵ����
��15�֣�ij��ѧ��ȤС����ʵ����̽�������й����ʣ��������ͼ��ʾʵ�飬A�������巢��װ�ã�A�����õ�ʵ��ҩƷ������������ѡȡ��
�� Na2CO3 �� NaOH �� Ca(OH)2 �� NaHCO3 �� NH4Cl
����ͼ���Ӻø��������ֽ�C���IJ�˿�����������ȣ��ٽ�A������������ͨ��Bװ��Ƭ�̺�ȥC���ƾ��ơ�����ʵ����������£���˿�������ֺ���״̬��D�е�ͭƬ�����ܽ⣬�ش��������⣺
��1��A�з����Ļ�ѧ��Ӧ����ʽΪ________����ȡ�������õ��IJ���������Ҫ��_______�֣�����������װ�ã���
��2��Bװ�õ�����Ϊ____________________________��
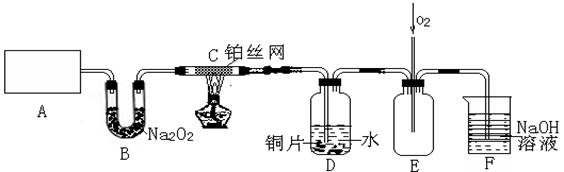 ��3��D��ͭƬ������Ӧ�����ӷ���ʽΪ___________________________��Ϊ��ʹCuƬ�ܽ�����ʼӿ죬����D������Һ�м����������������е�___________�������и�����ţ�
��3��D��ͭƬ������Ӧ�����ӷ���ʽΪ___________________________��Ϊ��ʹCuƬ�ܽ�����ʼӿ죬����D������Һ�м����������������е�___________�������и�����ţ�
A��Na2CO3 B��AgNO3 C��H2SO4 D��FeSO4
��4��ͼE�г���ͨ������������Ϊ____________________��
��5��������Ϊ������Ƶ�����ʵ��װ�û��������ԵIJ��㣬���䲻��֮���Լ�Ӧ��θĽ���̸̸��������_______________________________________________________��
��ͨ������˵�������ײ�ǵ�����ײ���Ƿǵ�����ײ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com