
����һƿʵ���ҷ����ѾõĿ��ܱ�������Na2SO3���壬Ϊ���о�������ɣ��������ͬѧ�ǽ��е�����̽�����
��ѡ���Լ���ŨH2SO4��ŨHNO3��10%���ᡢ0.1mol/LH2SO4��0.1mol/LHNO3��0.1mol/LBaCl2��0.1mol/LBa(NO3)2��3%H2O2��10%NaOH��Һ������ˮ��Ʒ����Һ��������ѡ��
��1���������
����һ������ȫ����Na2SO3�� �����������ȫ����Na2SO4��
�������� ��
��2�����ʵ�鷽��(��)��ѡ����ͼװ�ý���ʵ�飬��װ�õ��ŵ��� ��
��3������ʵ�飺�����±����ü�Ҫ����д��ʵ�������Ԥ������ͽ��ۡ�
| ʵ�鲽�� | Ԥ������ͽ��� |
| ����1��ȡ����������Ʒ�����Թ��У���W��a������ ��b������ ���ý��ܽ�W�������Թ����Ӻ� | |
| ����2������Ͳ���� ������ͷ�������ԹܵĽ������������Ʒ��ע�����Һ�� | �� |
| ����3��������Ͳ����������ˮϴ���������� ע�����Թ��� | �� |
��16�֣�
��1����2�֣�������Na2SO3��Na2SO4�Ļ����
��2����3�֣���ԼҩƷ��������Ⱦ��������ɫ��ѧ˼��
��3��ʵ�鲽�� Ԥ������ͽ��� ����1��ȡ����������Ʒ�����Թ��У���W��a����������Ʒ����Һ��1�֣���b����������10%NaOH��Һ��1�֣����ý��ܽ�W�������Թ����Ӻ� ����2������Ͳ��������10%������1�֣�������ͷ�������ԹܵĽ������������Ʒ��ע�����Һ�� ��2�֣��������ݷų���a��Ʒ����Һ��ɫ�������һ��������������������ݷų�����Ʒ�첻��ɫ�������������� ����3��������Ͳ����������ˮϴ��������������0.1mol/LBaCl2��Һ��1�֣�ע�����Թ��� ��2�֣������ְ�ɫ��������ϲ���2��Ʒ����ɫ�����������������δ���ְ�ɫ������ϲ���2��Ʒ����ɫ�������һ������
��4��SO2+H2O+Ca2+ + ClO�� = CaSO4��+2H+ +Cl����3�֣�
���������������1���������ƾ��л�ԭ�ԣ����ױ������е���������Ϊ�����ƣ���2Na2SO3+O2=2Na2SO4�����ݼ���һ��������û�б�����������������������������ȫ��������������ɴ��Ƶ������������������Ʋ��ֱ��������������������Na2SO3��Na2SO4�Ļ�����2������������Ҫ�ŵ��ǽ�ԼҩƷ��������Ⱦ��������ɫ��ѧ˼�룻��3����ͼ��W����a���Լ���Ŀ���Ǽ�������������壬 b���Լ���Ŀ�������ն���Ķ����������壬��ֹ��Ⱦ��������Ϊ���������ж��д̼�����ζ�����ڶ����������Ư���ԣ���ʹƷ����Һ��ɫ�����ڶ����������������������NaOH��Һ���ն���Ķ����������壬������Ŀ�Լ�Ҫ����1Ϊ��ȡ����������Ʒ�����Թ��У���W��a����������Ʒ����Һ��b����������10%NaOH��Һ���ý��ܽ�W�������Թ����Ӻã����Թ��Ƕ�������ķ���װ�ã�����Ӧװ�������������ƹ�����Ʒ����������������Ա���������Ҳ�����������������к�����������ӣ��������һ��ʵ�飩�������Ͳ��Ӧ�����������ᣬ����2Ϊ������Ͳ�������10%���ᣬ����ͷ�������ԹܵĽ������������Ʒ��ע�����Һ���������ݷų���a��Ʒ����Һ��ɫ�������һ��������������������ݷų�����Ʒ�첻��ɫ���������������������������Թ����Ƿ�����������ӣ���������������뱵���ӽ�����ɰ�ɫ���������������Ѿ�ʹ�������������ȫ��Ϊ�����������壬���������������ӵļ��飬���Բ���3��Ӧ���뼸�λ������Ȼ�����Һ���飬��������Ͳ����������ˮϴ��������������0.1mol/LBaCl2��Һ��ע�����Թ��У������ְ�ɫ��������ϲ���2��Ʒ����ɫ�����������������δ���ְ�ɫ������ϲ���2��Ʒ����ɫ�������һ������
��4������������л�ԭ�ԣ�Ư���д��������Һ����ǿ�����ԣ����߽�ǰ������Ϊ��������ӣ�ǰ�߽�����ԭΪ�����ӣ����������������ӽ�����ɰ�ɫ���������ݵ��ӡ���ɺ�ԭ���غ�ԭ���ɵã�SO2+H2O+Ca2+ + ClO�� = CaSO4��+2H+ +Cl����
���㣺���黯ѧʵ�鷽������������ۣ��漰Ԫ�ػ��������Ҫ���ʡ�̽��ʵ������������ļ��衢���巢������β������װ��ͼ�����ۡ����ʳɷ�̽��ʵ�鷽������ơ����ֽⷴӦ��������ԭ��Ӧ�����ӷ���ʽ�ȡ�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
��14�֣�
ij��ѧ��ȤС��Ϊ̽��Cl2��Br2��Fe3+��������ǿ�������������ʵ�飺
��1�� �ټ�����巢��װ��A�������ԵIJ����ǣ��ߣߣߣߣߣߣߣߣߣߣߣ�
������ʵ��װ�ô���һ�����Բ��㣬��ָ���ߣߣߣߣߣߣߣߣߣߣߣߣߣ�
��2�� �ø������װ�ý���ʵ�飮ʵ��������£�
| ʵ����� | ʵ������ | ���� |
| ����a����Բ����ƿ�е�������Ũ���Ȼ��رջ���a����ȼ�ƾ��� | Dװ���У���Һ��� Eװ���У�ˮ����Һ�����CCl4�������Ա仯 | Cl2��Br2��Fe3+����������ǿ������˳��Ϊ�� ______________________ |
| ��SCN��2������±�ص������ƣ������ԣ�Cl2����SCN��2�� ��Cl2��Br2��Ӧ����BrCl�����ʺ�ɫ���Դ���ɫ�����е�Ϊ5�棬��ˮ����ˮ�ⷴӦ�� ��AgClO��AgBrO��������ˮ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
ijͬѧ����������ⶨ������þ�����ᷴӦ�ⶨ1 mol������������������գ�
��1��A�з�����Ӧ�����ӷ���ʽΪ______________________��
��2�����װ�������Եķ���������Bƿ�IJ�����������Ƥ������Aƿ���Ͽڣ�������__________________����ʱ������ȷ��װ�����������á�
��3����֪Һ����ƿ�Ŀ̶ȷ�Χ��110~130 mL��ʵ��ʱ��ȡþ��������Ҫ������0.100~0.110 g֮�䣬Ŀ����_________��
��4�����һ�βⶨʵ�飬��Ҫ2����ע����������������Ҫ��¼���ǵ�__________�γ������������
��5����������ᵼ��ʵ����ƫ�ߵ���______�����ţ�
a. þ���������Ĥû�г��� b. ��Һƿ�е�Һ����ˮ
c. δ��ȴ�����¾Ͷ��� d. װ�������Բ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
ʵ������Ũ������ͭ�ķ�Ӧ��ȡ����NaHSO3��ʵ��װ����ͼ��ʾ��
��1��װ���ҵ�������_____________________��
��2��װ�ö���������������Ⱦ������SO2���壬�䷴Ӧ�����ӷ���Ϊ ��
��3�� SO2 ������Ư���ԡ���ԭ�Ժ������ԡ���SO2 ͨ����ˮ�У�SO2���ֵ���________�ԣ���ѧ��Ӧ����ʽΪ ��
��4����Ӧ�����У������еι����Ʒ����Һ������ƿ��������Ϊ ������Һ�е�NaOH��ȫת��Ϊ��NaHSO3��
��5��������û�м���Ʒ����Һ������ȷ�ж����������Ƿ���ȫת�������пɹ�ѡ����������Լ����ձ����Թܡ�����������ͷ�ιܣ� 2 mol/L���ᡢ2 mol/L���ᡢ1 mol/L�Ȼ�����Һ��l mol/L����������Һ��Ʒ����Һ������ˮ��
�����ʵ��̽�����պ�������Ƿ����NaHSO3 ��Na2SO3����ʵ�������Ԥ�ڵ�ʵ������ͽ��������±��С�
| ʵ����� | Ԥ����������� |
| ����1��ȡ��������Һ�����Թ��У��μӹ���lmol/L�Ȼ�����Һ������һ��ʱ��õ���ҺA����B�� | |
| ����2��������B�м�������ˮϴ�ӳ��������ú���ȥ�ϲ���Һ����������2�Σ���������Ʒ�죬�� | ��Ʒ����ɫ���������ݣ����� |
| ����3�� | �� �� �� ���� �� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
ij��ѧС��Ϊ��֤��SO2��Cl2��Ư���ԣ����������ͼ��ʾ��ʵ��װ�ã�
A B C D E
��1��ʵ�����Ʊ�Cl2���ݵ�ԭ���ǣ���ѧ����ʽ���� ��Ӧѡ����ͼA��Eװ���е� ������ţ���Cl2����Ӧ��Ũ���������ֳ��������� �� ��
��2����Ӧ��ʼ����B��D�����Թ��е�Ʒ����Һ����ɫ��ֹͣͨ����B��D�����Թ��е�Һ����ȣ�B�Թ��е������� ��
��3��NaOH��Һ��Cl2��Ӧ�����ӷ���ʽ�� ��
��4����С��ͬѧ�����������Ϻ�ͨ��Ʒ����Һ��һ��ʱ���Ʒ����Һ��������ɫ���������ϵ�֪���������尴�����1:1��ϣ�����ˮ��Ӧ���������ֳ������ᣬ���ʧȥƯ�����ã��÷�Ӧ�Ļ�ѧ����ʽ�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
��1��������ͭ������ᾧˮ�����IJⶨʵ���У�������������Ҫ����
�Ρ�
��2���к��Ȳⶨ��ʵ���У��õ��IJ����������ձ����¶ȼơ� �� ��
��3����������ѧ��ѧ�г��ò���������ɵ�ʵ��װ��ͼ(������Ҫ�������м���Һ������)��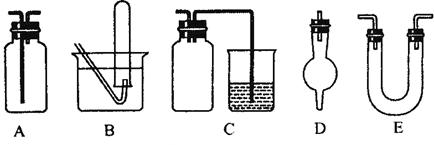
��ش��������⣺
�����������ﰱ����װ����_______________(����ĸ)��
�ڼ��������ռ��������������ռ�һ�����������װ����_______________(����ĸ)��
����ʵ�����Ʊ�������ʵ���У����Գ�ȥ�������Ȼ�������������װ����________________ (����ĸ)��
����������ϩ����ˮ��Ӧ�ƶ��������ʵ��װ����__________(����ĸ)��
����Cװ���У������ձ��ڵ�����������Һ����β��������������ƿ��������___________________��
��4��Ϊ�Ƚ�Fe3+��Cu2+��H2O2�ֽ�Ĵ�Ч����ijͬѧ�������ͼ��ʾ��ʵ�顣
�ٿ�ͨ���۲� �����Եĵó����ۣ�
����Aͬѧ�����CuSO4��ΪCuCl2��Ϊ�������������� ��
��������Aͬѧ�ĸĽ�����������Ϊ��������θĽ��� ��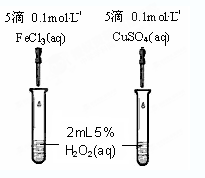
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ʴ���
( 14��)ij��ѧС�����Na2O2��ˮ��Ӧ��ʵ�飬����ͼ��ʾ����С����Թ�c�к�ɫ��ȥ��ԭ�����̽����
��1��Na2O2�к��еĻ�ѧ�����ͣ� ����д��a�з�Ӧ�Ļ�ѧ����ʽ ��
�������ϣ�
�ٵ�NaOH��ҺpH��13ʱ������ʹ��̪�ɺ�ɫ��Ϊ��ɫ��
��Na2O2��ˮ��Ӧ���������У�Na2O2 + H2O =" NaOH" + H2O2 2H2O2 = 2H2O + O2��
��2�������ʵ����֤Na2O2��ˮ��Ӧ�����Һ����H2O2������ȡ����b��Һ���Թ��У� ��֤����Һ����H2O2������
��3��������ϣ���С��ͬѧ��c����Һ��ɫ��ȥ��ԭ��������¼��裺
�� ��
�� ��Һ��H2O2�ƻ���̪�Ľṹ��
�� NaOH��H2O2��ͬ���ý����
��4����С��ͬѧ���c����Һ��pHΪ14����Ϊ�������ų�����ڡ��ۣ������ֽ���������ʵ�飬������±��հ״���
| ʵ�� | ���� | ���� | ���� |
| 1 | ������H2O2�еμ�2�η�̪������һ��ʱ�䣬�ټ���NaOH��Һ��pH=12 | ����NaOH����ɫ��Һ�ȱ�죬����ɫ | �� |
| 2 | ������ NaOH��Һ��pH=14���еμ�2�η�̪���ټ�����ϡ��������Һ pH=12 | ��Һ�ȱ�죬����ɫ����������ֳ��ֺ�ɫ���Ҳ���ɫ |  |
| 3 | ��Na2O2��ˮ��Ӧ�����Һ��pH=14���еμ�2�η�̪���ټ�����ϡ��������Һ pH=12 ���� | �� ���� | ��ҺpH����13ʱ��NaOHʹ������Һ��ɫ��pH��8~13ʱ�� NaOH��H2O2��ͬ����ʹ��Һ��ɫ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
Ϊ��̽��SO2��Na2O2�ķ�Ӧ�Ƿ�������CO2����ͬѧ�������ͼʵ��װ�á��ش��������⣺
��1���ƿ������������ǵ�ľ������C�Թܿڣ�δ��ľ����ȼ����ͬѧ�����ΪSO2��Na2O2�ķ�Ӧ��ͬ��CO2���밴��ͬѧ�Ĺ۵�д����Ӧ�Ļ�ѧ����ʽ ��
��2����ͬѧ��Ϊ���۷�Ӧԭ����Σ����ն���O2��������ͬѧ�������� ��
������ͬѧ�Ĺ۵㣬��װ�������ĸĽ��ǣ� ��
��3������Na2O2 ��ȫ��Ӧ����Ӧ��Bװ���й�������������ǣ�
�� Na2SO3�� �� Na2SO4�� �� Na2SO3��Na2SO4
�����ʵ�鷽�����飬д��ʵ�鲽���Լ�Ԥ������ͽ��ۣ�����±���
��ѡ�Լ���2 mol��L��1 HCl�� 1mol��L��1HNO3�� 1 mol��L��1 BaCl2�� 1 mol��L��1 Ba(NO3)2��
0.01mol��L��1KMnO4������Һ��
| ʵ�鲽�� | Ԥ������ͽ��� |
| ����1��ȡB�е�����������Ʒ���Թ��У��μ���������ˮ���ܽ⣬Ȼ��ȡ��������Һ�ֱ����ڢ��Թ��С� | ������ȫ�ܽ� |
| ����2�������Թ��м��� ���ٵμ� �� | ����֤������ ���к�Na2SO4�� |
| ����3�������Թ��� �� | �� , ��˵������������Na2SO3���� , ��˵����������û��Na2SO3�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
������л�ѧ������˵����ȷ����
| A������60Co�ķ����Կ�����ijЩ������60Co��59Co��Ϊͬλ�� |
| B��������ˮ�� ��84�� ����Һ������ԭ����ͬ |
| C��̼�ᱵ�����ᱵ�������Ա��ξ������ڱ����� |
| D���±���װ���г���������������� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com