
| A�� | ��Ԫ����������Һ������ɫ | |
| B�� | ������Ϊ86��������Ϊ51���ԭ�ӿɱ�ʾΪ��$\stackrel{137}{86}$Rn | |
| C�� | CS2�ĵ���ʽ����$\underset{\stackrel{..}{S}}{..}$��$\underset{\stackrel{..}{C}}{..}$��$\underset{\stackrel{..}{S}}{..}$�� | |
| D�� | 6.0g���ᾧ���к���H+����ĿΪ0.1NA |
���� A���������ⵥ�ʱ���ɫ��
B��������+������=�����������ԭ�ӱ�ʾ��ʽ������
C��CS2�Ľṹ������CO2�Ľṹ����ԭ����̼ԭ��֮���γ�2�Թ��õ��Ӷԣ�
D�������ǹ��ۻ�������ᾧ�岻�������ӣ�
��� �⣺A������ֻ�������ⵥ�ʲű���ɫ���ɵ�Ԫ�ع��ɵ��������ʲ���ʹ���۱���ɫ����A����
B��������Ϊ86��������Ϊ51��������=86+51=137���ԭ�ӿɱ�ʾΪ��$\stackrel{137}{86}$Rn����B��ȷ��
C����ԭ����̼ԭ��֮���γ�2�Թ��õ��Ӷԣ�����ʽΪ ����C����
����C����
D�������ǹ��ۻ����ֻ������Һ�в��ܵ���������ӣ�������ʱ���������ӣ���D����
��ѡB��
���� ���⿼���˵��۵����ԡ�ԭ�ӹ��ɡ�����ʽ����д��֪ʶ���漰֪ʶ���ɢ��Ϊ�߿��������ⷽʽ���ѶȲ���ע����ᾧ��ʹ�����Һ�IJ��


 ��У����ϵ�д�
��У����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
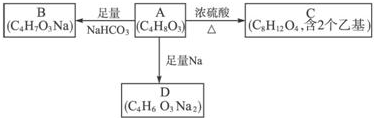
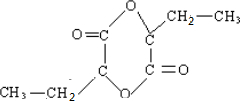 ��
��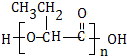 ��
���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | CH3CH2Br+NaHS��CH3CH2SH+NaBr | |
| B�� | CH3I+CH3ONa��CH3OCH3+NaI | |
| C�� | CH3CH2Cl+CH3ONa��CH3CH2ONa+CH3Cl | |
| D�� | CH3CH2Cl+CH3CH2ONa����CH3CH2��2O+NaCl |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
| �ζ����� | NaOH��Һ���/mL | �ζ�������Һ���/mL |
| 1 | 25.00 | 20.02 |
| 2 | 25.00 | 17.10 |
| 3 | 25.00 | 19.98 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �����ͼ���ϡ������������ | |
| B�� | ��Ȼ����Һ��ʯ�����ijɷ���ͬ | |
| C�� | ��ϩ����ȩ���ܷ����ӳɷ�Ӧ | |
| D�� | ʯ���ѽ������ˮ�ⶼ���ɸ߷�������С���ӵĹ��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ̼�������ɰֽ��ɰ�ֵ�ĥ�� | |
| B�� | �ع���û�����ü�ֵ������ȼ�շ����� | |
| C�� | ʳ�Ρ�ʳ�ס����С�մ��dz����ij�����Ʒ | |
| D�� | ����������̫�����ǽ�Լ��ʯ��Դ���ֲ���Դ�����;�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �ú������۵���С����ʳƷһ���ܷ��װ��˵������������ˮ�� | |
| B�� | Ũ�������ʢ������Ͱ�У�˵����������Ũ���ᷴӦ | |
| C�� | ��������̼��������������ĭ��������������������Һ��̼��������Һ��Ϻ��ܷ�������˫ˮ�ⷴӦ | |
| D�� | ��������̲�����˵���������ǿ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
| A�� | 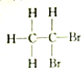 �� �� | B�� | 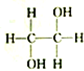 �� �� | C�� | 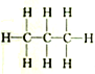 �� ��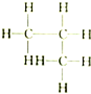 | D�� | CH3-CH2-CHO��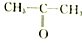 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | Tl+���������1������ | B�� | Tl���γ�+3�ۺ�+1�۵Ļ����� | ||
| C�� | ������Һ��Tl3+��Tl+������ǿ | D�� | Tl+�Ļ�ԭ�Ա�Ag�� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com