
(1)KBrO3�ܽ�KI������I2��KIO3���䱾������ԭΪBr2��
(2) Br2�ܽ�I-����ΪI2��
(3)KIO3�ܽ�I-����ΪI2��Ҳ�ܽ�Br-����ΪBr2���䱾������ԭΪI2��
��������(Br2��KBrO3��KlO3)����������ǿ������˳��Ϊ��____________________��
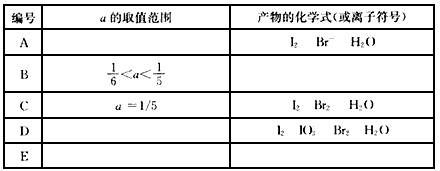
��������1molKI��������Һ�м��뺬amolKBrO3��Һ��a��ȡֵ��ͬ�����ò���Ҳ��ͬ���Խ����۵Ľ�������±��У�
��� | a��ȡֵ��Χ | ����Ļ�ѧʽ(�����ӷ���) |
A |
| I2 Br- H2O |
B |
|
|
C | a=1/5 | I2 Br2 H2O |
D |
| I2 |
E |
|
|
���������еⵥ�ʺ��嵥�ʵ����ʵ������ʱ��a��ֵΪ��_____________��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����

| 1120(W2-W1) |
| 160a |
| 1120(W2-W1) |
| 160a |

| 14bc |
| 5a |
| 14bc |
| 5a |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ҵ�ϴӺ�ˮ��ȡ�壬�漰����Ũ��������������������ȡ���Ȳ��裬�����ڡ���ȡ�������У����ÿ������崵����Ȼ����̼������Һ���գ���ʱ��ת��ΪBr�����Ӻ�BrO3�����ӣ�ͬʱ�ж�����̼�������ɡ�����������ữ���������ִ���Һ��������
��1��д����������̼������Һ��Ӧ�Ļ�ѧ����ʽ________________________________��
��2���������ữʱ��ÿת��1 mol���ӣ��������ɵ�����___________ mol��
��3����֪�����������������·�Ӧ��ϵ��
��KBrO3�ܽ�KI������I2 ��KIO3���䱾������ԭΪBr2��
��Br2�ܽ�I?? ����ΪI2��
��KIO3�ܽ�I??����ΪI2��Ҳ�ܽ�Br��������Br2���䱾������ԭΪI2��
Br2��BrO3����I2��IO3������������ǿ������˳��Ϊ__________________________��
��4���������ӷ���ʽ�������______________(ѡ��𰸱��)��
A��IO3��+ Br����I��+ BrO3��
B��6I��+ BrO3��+ 6H + �� 3I2 + Br��+ 3H2O
C��5I��+ 6BrO3��+ 6H + ��3 Br2+ 5 IO3��+ 3H2O
D��5I��+ 2BrO3��+ 6H + �� Br2 + IO3��+ 2I2 + 3H2O
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��ר���� ���ͣ������
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com