
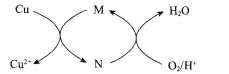
”¾ĢāÄæ”æĖ«¼«Ä¤µēÉųĪöŅ»²½·ØŃĪÖĘĖį¼īµÄ¼¼Źõ½ųČėµ½ĮĖ¹¤Ņµ»Æ½×¶Ī£¬Ä³æĘŃŠŠ”×éŃŠ¾æ²ÉÓĆBMEDĤ¶Ń(ČēĶ¼ĖłŹ¾)£¬Ä£ÄāŅŌ¾«ÖĘÅØŗ£Ė®ĪŖŌĮĻÖ±½ÓÖʱøĖįŗĶ¼ī”£BMEDĤ¶Ń°üĄØŃōĄė×Ó½»»»Ä¤”¢ŅõĄė×Ó½»»»Ä¤ŗĶĖ«¼«Ä¤(a”¢d)ŅŃÖŖ£ŗŌŚÖ±Į÷µēŌ“µÄ×÷ÓĆĻĀ£¬Ė«¼«Ä¤ÄŚÖŠ¼ä½ēĆę²ć·¢ÉśĖ®µÄ½āĄė£¬Éś³ÉH+ŗĶOH-”£ĻĀĮŠĖµ·ØÕżČ·µÄŹĒ

A. µē¼«YĮ¬½ÓµēŌ“µÄÕż¼«£¬·¢Éś»¹Ō·“Ó¦
B. IæŚÅųöµÄŹĒ»ģŗĻ¼ī£¬¢ņæŚÅųöµÄŹĒµĖ®
C. µē½āÖŹČÜŅŗ²ÉÓĆNa2SO4ČÜŅŗæɱÜĆāÓŠŗ¦ĘųĢåµÄ²śÉś
D. a×ó²ąÄ¤ĪŖŃōĄė×Ó½»»»Ä¤£¬cĪŖŅõĄė×Ó½»»»Ä¤
”¾“š°ø”æC
”¾½āĪö”æ
A.ĒāŃõøłĄė×ÓĻņ×ó²ąŅĘ¶Æ£¬ÕāĖµĆ÷µē¼«YĪŖŅõ¼«£¬ĖłŅŌµē¼«YĮ¬½ÓµēŌ“µÄøŗ¼«£¬·¢Éś»¹Ō·“Ó¦£¬¹ŹA“ķĪó£»
B.ÅØŗ£Ė®ÖŠµÄĀČĄė×ÓĻņ×ó²ąŅĘ¶Æ£¬ÄĘĄė×ÓĻņÓŅ²ąŅĘ¶Æ£»Ė«¼«Ä¤ÖŠ£¬ĒāĄė×ÓĻņÓŅ²ąĒØŅĘ”¢ĒāŃõøłĄė×ÓĻņ×ó²ąĒØŅĘ£¬Ņņ“Ė¢ņæŚÅųöµÄŹĒµĖ®£¬IæŚÅųöµÄŹĒŃĪĖį”¢¢óæŚÅųöµÄŹĒ¼īŅŗ£¬¹ŹB“ķĪó£»
C.ÓÉÓŚĀČĄė×ӷŵē»į²śÉśÓŠ¶¾µÄĘųĢåĀČĘų£¬¼ÓČėNa2SO4ČÜŅŗ£¬ÄæµÄŹĒŌö¼ÓČÜŅŗµÄµ¼µēŠŌ£¬ĀČĄė×ÓŅĘĻņ¢ńŹŅ£¬ĒāĄė×ÓĶعżaŅĘĻņ¢ńŹŅ£¬ŌŚ¢ńŹŅµĆµ½HCl£¬æɱÜĆāÓŠŗ¦ĘųĢåµÄ²śÉś£¬¹ŹCÕżČ·£»
D.ÄĘĄė×ÓŅĘĻņ¢óŹŅ£¬cĪŖŃōĄė×Ó½»»»Ä¤£¬ĒāŃõøłĄė×ÓĻņ×ó²ąŅĘ¶Æ£¬ĖłŅŌa×ó²ąÄ¤ĪŖŅõĄė×Ó½»»»Ä¤£¬¹ŹD“ķĪó”£
¹ŹŃ”C”£


 ĘŚÄ©ŗĆ³É¼ØĻµĮŠ“š°ø
ĘŚÄ©ŗĆ³É¼ØĻµĮŠ“š°ø 99¼Ó1ĮģĻČĘŚÄ©ĢŲѵ¾ķĻµĮŠ“š°ø
99¼Ó1ĮģĻČĘŚÄ©ĢŲѵ¾ķĻµĮŠ“š°ø °ŁĒæĆūŠ£ĘŚÄ©³å“Ģ100·ÖĻµĮŠ“š°ø
°ŁĒæĆūŠ£ĘŚÄ©³å“Ģ100·ÖĻµĮŠ“š°ø ŗĆ³É¼Ø1¼Ó1ĘŚÄ©³å“Ģ100·ÖĻµĮŠ“š°ø
ŗĆ³É¼Ø1¼Ó1ĘŚÄ©³å“Ģ100·ÖĻµĮŠ“š°ø ½š×“ŌŖ¼ØÓÅŗĆ¾ķĻµĮŠ“š°ø
½š×“ŌŖ¼ØÓÅŗĆ¾ķĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æ¼īŹ½ĀČ»ÆĶ[Cux(OH)yClz£®mH2O]ŹĒÖŲŅŖµÄÅ©Ņ©”¢Ņ½Ņ©ÖŠ¼äĢ壬»¹æÉÓĆ×÷ľ²Ä·ĄøƼĮ”¢ĖĒĮĻĢķ¼Ó¼ĮµČ”£ŃŠ¾æŠ”×éŌŚŹµŃéŹŅÓĆij³§·ĻĶŌü(Ö÷ŅŖ³É·ÖCu”¢CuO£¬ŗ¬ÉŁĮæFe3O4”¢Ni”¢A12O3)Öʱø¼īŹ½ĀČ»ÆĶµÄĮ÷³ĢČēĻĀ£ŗ
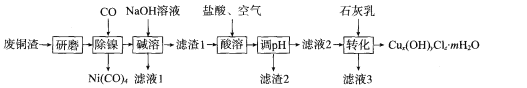
»Ų“šĻĀĮŠĪŹĢā£ŗ
(l)”°ŃŠÄ„”±µÄÄæµÄĪŖ___ ”£
(2)”°¼īČÜ”±µÄÄæµÄĪŖ __ ”£
(3)”°ĖįČÜ”±Ź±Éś³ÉFe3+·“Ó¦µÄĄė×Ó·½³ĢŹ½ĪŖ ___£»Éś³ÉµÄFe3+¶ŌCu·¢ÉśµÄŃõ»Æ·“Ó¦µÄ“ß»ÆŌĄķČēĶ¼ĖłŹ¾”£NµÄ»ÆѧŹ½ĪŖ____”£
(4)”°ĖįČÜ”±Ź±ĪĀ¶Č²»ÄܹżøߵĥķÓÉĪŖ____”£
(5)ČōĀĖŅŗ2ÖŠc(Fe3+)=4”Į10-8 mol/L£¬pH=4£¬ŌņKsp[Fe(OH)3]=____”£
(6)ĪŖ²ā¶ØCux (OH)yClz£®mH2OµÄ×é³É£¬½ųŠŠČēĻĀ²Ł×÷£ŗȔѳʷ2.232 g£¬ÓĆŹŹĮæĖįČܽāŗóÅä³É100 mLČÜŅŗ£»Č”25. 00 mLČÜŅŗ¼ÓČė×ćĮæAgNO3ČÜŅŗ£¬Éś³É0.3444 g³Įµķ£»ĮķČ”25. 00 mLČÜŅŗ£¬ÓĆ0.1600 mol£®L-1µÄEDTA±ź×¼ŅŗµĪ¶ØCu2+(Cu2+ÓėEDTAŅŌĪļÖŹµÄĮæÖ®±Č1£ŗ1·“Ó¦)£¬µĪ¶ØÖĮÖÕµćŹ±Ļūŗıź×¼ŅŗĢå»żĪŖ30. 00 mL”£
¢ŁČܽāѳʷĖłÓĆĖįµÄ»ÆѧŹ½ĪŖ ___”£
¢ŚøĆѳʷµÄ»ÆѧŹ½ĪŖ____”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æŗ£“ųÖŠŗ¬ÓŠ·įø»µÄµā”£ĪŖĮĖ“Óŗ£“ųÖŠĢįČ”µā£¬Ä³ŃŠ¾æŠŌѧĻ°Š”×éÉč¼Ę²¢½ųŠŠĮĖŅŌĻĀŹµŃé£ŗ

ĒėĢīŠ“ĻĀĮŠæÕ°×£ŗ
£Ø1£©²½Öč¢Ł×ĘÉÕŗ£“ųŹ±£¬Ź¢·Åŗ£“ųµÄŅĒĘ÷Ćū³ĘŹĒ______________”£
£Ø2£©²½Öč¢ŪµÄŹµŃé²Ł×÷Ćū³ĘŹĒ_________£¬²½Öč¢ŽµÄÄæµÄŹĒ“Óŗ¬µā±½ČÜŅŗÖŠ·ÖĄė³öµāµ„ÖŹŗĶ»ŲŹÕ±½£¬øĆ²½ÖčµÄŹµŃé²Ł×÷Ćū³ĘŹĒ_______________”£
£Ø3£©²½Öč¢Ü·“Ó¦µÄĄė×Ó·½³ĢŹ½ŹĒ______________________________”£
£Ø4£©ĒėÉč¼ĘŅ»ÖÖ¼ģŃéĢįČ”µāŗóµÄĖ®ČÜŅŗÖŠŹĒ·ń»¹ŗ¬ÓŠµ„ÖŹµāµÄ¼ņµ„·½·Ø___________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æČēĶ¼ĪŖÓÉŹÆÓĶÖĘČ”ĘūÓĶµÄ×°ÖĆŹ¾ŅāĶ¼£¬øł¾ŻĶ¼Ź¾»Ų“šĻĀĮŠĪŹĢā”£

(1)Ķ¼ÖŠµÄĮ½“¦Ć÷ĻŌµÄ“ķĪóŹĒ________________”¢__________________
(2)AŅĒĘ÷µÄĆū³ĘŹĒ________£¬BŅĒĘ÷µÄĆū³ĘŹĒ________”£
(3)ŹµŃ鏱 A ÖŠ³ż¼ÓČėŹÆÓĶĶā£¬»¹Šč¼ÓČėÉŁĮæ__________£¬Ęä×÷ÓĆŹĒ__________________”£
(4)ŹÕ¼ÆĶźĘūÓĶŗó£¬ŹĒĻČ³·¾Ę¾«µĘ»¹ŹĒĻČĶ£ĄäÄżĖ®£æ
______________________________________________________________
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æĪļÖŹCŹĒÉżøß°×Ļø°ūµÄ³£¼ūŅ©ĪļÉč¼ĘŗĻ³ÉCµÄĀ·ĻßČēĻĀĶ¼ĖłŹ¾£ŗ

ŅŃÖŖ:

Ēė»Ų“šĻĀĮŠĪŹĢā£ŗ
(1)¢ŁµÄ·“Ó¦Ģõ¼žĪŖ____;AÖŠ¹ŁÄÜĶŵÄĆū³ĘŹĒ_____”£
(2)ŹŌ¼ĮXµÄ½į¹¹¼ņŹ½ĪŖ____,¢ÜµÄ»Æѧ·½³ĢŹ½ĪŖ________,Ęä·“Ó¦ĄąŠĶĪŖ_____”£
(3)ĪļÖŹBÓėH2°“ÕÕĪļÖŹµÄĮæÖ®±Č1:1¼Ó³ÉµĆµ½ĪļÖŹT”£Š“³öŅ»ÖÖ·ūŗĻĻĀĮŠĢõ¼žµÄTµÄĶ¬·ÖŅģ¹¹ĢåµÄ½į¹¹¼ņŹ½____________”£
¢ŁŹōÓŚ·¼Ļć×å»ÆŗĻĪļ
¢ŚlmoløĆĪļÖŹÓė×ćĮæĢ¼ĖįĒāÄĘČÜŅŗ·“Ӧɜ³É2 mol CO2
¢ŪŗĖ“Ź²ÕńĒāĘ×ÓŠ2×éĪüŹÕ·å,ĘäĆ껿±ČĪŖ6:2
(4)ŅŌR”å”ŖCHO“ś±ķXŠ“³öXÓė±½·ÓŌŚŅ»¶ØĢõ¼žĻĀŠĪ³Éøß¾ŪĪļµÄ»Æѧ·½³ĢŹ½____”£
(5)Éč¼ĘŅŌ¼×Č©”¢ŅŅČ©ĪŖŌĮĻŗĻ³É µÄĀ·Ļß(ĪŽ»śŹŌ¼ĮČĪŃ”)_____________”£
µÄĀ·Ļß(ĪŽ»śŹŌ¼ĮČĪŃ”)_____________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æĻĀĮŠÓŠ¹ŲĖµ·ØÕżČ·µÄŹĒ£Ø””””£©
A. ±½·ÓÄĘČÜŅŗÖŠĶØČėÉŁĮæµÄ¶žŃõ»ÆĢ¼£¬²śĪļŹĒ±½·ÓŗĶĢ¼ĖįÄĘ
B. ±½µÄĶ¬ĻµĪļÖŠ£¬±½»·ŗĶ²ąĮ“Ļą»„Ó°Ļģ£¬Ź¹µĆ¶žÕß¾łŅ×±»Ńõ»Æ
C. ![]() ŌŚŗĖ“Ź²ÕńĒāĘ×ÖŠ³öĻÖĮ½×é·å£¬ĘäĒāŌ×ÓŹżÖ®±ČĪŖ3£ŗ2
ŌŚŗĖ“Ź²ÕńĒāĘ×ÖŠ³öĻÖĮ½×é·å£¬ĘäĒāŌ×ÓŹżÖ®±ČĪŖ3£ŗ2
D. ±½¼×Č©”¢±½ŅŅĻ©·Ö×ÓÖŠµÄĖłÓŠŌ×ÓæÉÄÜ“¦ÓŚĶ¬Ņ»Ę½Ćę
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æČēĶ¼ĖłŹ¾£¬Ņ»ĆܱÕČŻĘ÷±»ĪŽÄ¦²Į”¢æÉ»¬¶ÆµÄĮ½øō°åa”¢b·Ö³É¼×”¢ŅŅĮ½ŹŅ£»±ź×¼×“æöĻĀ£¬ŌŚŅŅŹŅÖŠ³äČėNH3 0.4 mol£¬¼×ŹŅÖŠ³äČėHClŗĶN2µÄ»ģŗĻĘųĢ壬¾²Ö¹Ź±øō°åĪ»ÖĆČēĶ¼ĖłŹ¾”£ŅŃÖŖ¼×”¢ŅŅĮ½ŹŅÖŠĘųĢåµÄÖŹĮæ²īĪŖ17.3g£¬Ōņ¼×ŹŅÖŠHClŗĶN2µÄĪļÖŹµÄĮæÖ®±ČĪŖ£Ø £©

A.1”Ć3B.1”Ć4C.3”Ć1D.4”Ć1
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æÓĆNA±ķŹ¾°¢·ü¼ÓµĀĀŽ³£ŹżµÄÖµ”£ĻĀĮŠŠšŹöÕżČ·µÄŹĒ

A. 17gōĒ»ł(£OH)Ėłŗ¬µē×Ó×ÜŹżĪŖ9NA
B. 18gD2OÖŠŗ¬ÓŠµÄŌ×ÓŹżĪŖ3NA
C. ±ź×¼×“æöĻĀ£¬22.4LCHCl3ÖŠĖłŗ¬·Ö×ÓŹżĪŖNA
D. 32g S8µ„ÖŹ(½į¹¹ČēĶ¼)ÖŠŗ¬ÓŠµÄS£S¼üøöŹżĪŖ2NA
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æ³£¼ūµÄĪåÖÖŃĪA£¬B£¬C£¬D£¬E£¬ĖüĆĒµÄŃōĄė×ÓæÉÄÜŹĒNa£«”¢NH4+£¬Cu2£«£¬Ba2£«”¢Al3£«”¢Ag£«”¢Fe3£«£¬ŅõĄė×ÓæÉÄÜŹĒCl£”¢NO![]() ”¢SO
ӢSO![]() ӢCO
”¢CO![]() ”£ŅŃÖŖ£ŗ
”£ŅŃÖŖ£ŗ
¢ŁĪåÖÖŃĪ¾łČÜÓŚĖ®£¬Ė®ČÜŅŗ¾łĪŖĪŽÉ«”£
¢ŚDµÄŃęÉ«·“Ó¦³Ź»ĘÉ«”£
¢ŪAµÄČÜŅŗ³ŹÖŠŠŌ£¬B£¬C£¬EµÄČÜŅŗ³ŹĖįŠŌ£¬DµÄČÜŅŗ³Ź¼īŠŌ”£
¢ÜČōŌŚÕāĪåÖÖŃĪµÄČÜŅŗÖŠ·Ö±š¼ÓČėBa(NO3)2ČÜŅŗ£¬Ö»ÓŠA£¬CµÄČÜŅŗ²»²śÉś³Įµķ”£
¢ŻČōŌŚÕāĪåÖÖŃĪµÄČÜŅŗÖŠ£¬·Ö±š¼ÓČė°±Ė®£¬EŗĶCµÄČÜŅŗÖŠÉś³É³Įµķ£¬¼ĢŠų¼Ó°±Ė®£¬CÖŠ³ĮµķĻūŹ§”£
¢Ž°ŃAµÄČÜŅŗ·Ö±š¼ÓČėµ½B£¬C£¬EµÄČÜŅŗÖŠ£¬¾łÄÜÉś³É²»ČÜÓŚĻ”ĻõĖįµÄ³Įµķ”£
»Ų“šĻĀĮŠĪŹĢā£ŗ
(1)ĪåÖÖŃĪÖŠ£¬Ņ»¶ØƻӊµÄŃōĄė×ÓŹĒ________£»Ėłŗ¬µÄŅõĄė×ÓĻąĶ¬µÄĮ½ÖÖŃĪµÄ»ÆѧŹ½ŹĒ________”£
(2)DµÄ»ÆѧŹ½ĪŖ________£¬DČÜŅŗĻŌ¼īŠŌµÄŌŅņŹĒ(ÓĆĄė×Ó·½³ĢŹ½±ķŹ¾)__________________”£
(3)AŗĶCµÄČÜŅŗ·“Ó¦µÄĄė×Ó·½³ĢŹ½ŹĒ______________”£
EŗĶ°±Ė®·“Ó¦µÄĄė×Ó·½³ĢŹ½ŹĒ___________________”£
(4)ČōŅŖ¼ģŃéBÖŠĖłŗ¬µÄŃōĄė×Ó£¬ÕżČ·µÄŹµŃé·½·ØŹĒ_____________________
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com