
���� ��һ�ݷݼ������������ữ�����AgNO3��Һ�а�ɫ�������������������������ӣ����ж�ԭ��Һ���Ƿ���Cl-��
�ڶ�����Һ������NaOH��Һ���Ⱥ��ռ�����״����560ml���壬�ڴ˹�����û�г����������Ƶ�һ������NH4+��һ��������Mg2+�����ݷ�ӦNH4++OH-$\frac{\underline{\;\;��\;\;}}{\;}$NH3��+H2O��ÿ�ݺ���笠����ӵ����ʵ���Ϊ��$\frac{0.56L}{22.4L/mol}$=0.025mol��
�����ݼ�����BaCl2��Һ���ˡ�ϴ�ӡ�����õ�����8.6g��������������ϡ������ܽ⣬���ˡ�ϴ�ӡ������������Ϊ4.66g��������������ij���ΪBaCO3������������ij���ΪBaSO4��������ӦΪ��CO32-+Ba2+�TBaCO3����SO42-+Ba2+�TBaSO4������ΪBaCO3+2HCl�TBaCl2+CO2��+H2O��ʹBaCO3�ܽ⣬�����Һ��һ������CO32-��SO42-���������ӹ����֪ԭ��Һ��һ������Ba2+�����������غ��֪��ÿ����Һ�к��е�n��SO42-��=n��BaSO4��=$\frac{4.66g}{233g/mol}$=0.02mol��n��CO32-��=n��BaCO3��=$\frac{8.6g-4.66g}{197g/mol}$=0.02mol��
�����������ɵã���Һ��һ������CO32-��SO42-��NH4+��һ��������Mg2+��Ba2+����CO32-��SO42-��NH4+���ʵ����ֱ�Ϊ0.025mol��0.02mol��0.02mol��CO32-��SO42-�����ܵĸ���ɷֱ�Ϊ��0.02mol��2=0.02mol��2=0.08mol��NH4+���������Ϊ0.025mol���ٽ�ϵ���غ��ж��������ӵĴ��������
��� �⣺��һ�ݷ����������������ӣ����ж�ԭ��Һ���Ƿ���Cl-��
�ڶ�����Һ������NaOH��Һ�������ɵ�����Ϊ�������Ƶ�һ������NH4+������û�г������ɣ���һ��������Mg2+�����ݷ�ӦNH4++OH-$\frac{\underline{\;\;��\;\;}}{\;}$NH3��+H2O��ÿ�ݺ���笠����ӵ����ʵ���Ϊ��$\frac{0.56L}{22.4L/mol}$=0.025mol��
�����ݼ�����BaCl2��Һ���ˡ�ϴ�ӡ�����õ�����8.6g��������������ϡ������ܽ⣬���ˡ�ϴ�ӡ������������Ϊ4.66g��������������ij���ΪBaCO3������������ij���ΪBaSO4��������ӦΪ��CO32-+Ba2+�TBaCO3����SO42-+Ba2+�TBaSO4������ΪBaCO3+2HCl�TBaCl2+CO2��+H2O��ʹBaCO3�ܽ⣬�����Һ��һ������CO32-��SO42-���������ӹ����֪ԭ��Һ��һ������Ba2+�����������غ��֪��ÿ����Һ�к��е�n��SO42-��=n��BaSO4��=$\frac{4.66g}{233g/mol}$=0.02mol��n��CO32-��=n��BaCO3��=$\frac{8.6g-4.66g}{197g/mol}$=0.02mol��
�����������ɵã���Һ��һ������CO32-��SO42-��NH4+��һ��������Mg2+��Ba2+����CO32-��SO42-��NH4+���ʵ����ֱ�Ϊ0.025mol��0.02mol��0.02mol��CO32-��SO42-�����ܵĸ���ɷֱ�Ϊ��0.02mol��2=0.02mol��2=0.08mol��NH4+���������Ϊ0.025mol������һ�����������ӣ������ӵ����ʵ�����СΪ0.055mol�������Ӳ���ȷ����
��1��������ӵ������к��������ӣ���ԭ����Һ�е������ӵļ����и��ţ�������ȷ��ԭ��Һ���Ƿ��������ӣ�
�ʴ�Ϊ�����ܣ���ӵ������к��������ӣ���ԭ����Һ�е������ӵļ����и��ţ�
��2�����ݷ�����֪��ԭ���Һ��һ������NH4+��ÿ����Һ�к���0.025mol笠����ӣ���Ũ��Ϊ��$\frac{0.025mol}{0.1L}$=0.25mol/L��
�ʴ�Ϊ��NH4+��0.25mol/L��
��3�������ݽ��е�ʵ���֪8.6g�����ijɷ�ΪBaCO3��BaSO4��ԭ��Һ��һ������CO32-��SO42-��ÿ����Һ�к���0.02molCO32-��0.02molSO42-������Ũ�ȶ��ǣ�c=$\frac{0.02mol}{0.1L}$=0.2mol/L��
�ʴ�Ϊ��BaCO3��BaSO4��CO32-��SO42-��0.2 mol/L��0.2 mol/L��
��4�����ݷ�����֪��ÿ����Һ�����ٺ���0.055molNa+��Ũ��Ϊ��c��Na+��=$\frac{0.055mol}{0.1L}$0.55mol/L��
�ʴ�Ϊ��Na+��0.55mol/L��
���� ���⿼���˳������ӵļ��鷽������Ŀ�Ѷ��еȣ���ȷ�������ӵ�����Ϊ���ؼ���ע���������ճ������ӵļ��鷽�������ݵ���غ��ж������ӵĴ������Ϊ�״��㣬����������ѧ���ķ������������Ӧ��������



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | Na2CO3��H2SO4 | B�� | NaAlO2��HCl | C�� | Na2CO3��Ca��OH��2 | D�� | KAl��SO4��2��NaOH |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | NaOH��Һ | B�� | ��ˮ | C�� | CuO | D�� | KSCN��Һ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| ʵ�� | ���� | ���� | |
| A | �������ữ��H2O2����Fe��NO2��2��Һ | ��Һ���ɫ | H2O2�������Ա�Fe3+ǿ |
| B | �����pH=2��HX��HY������ֱ�������������Ӧ����ˮ���ռ����� | HX�ų��������� | HX���Ա�HY�� |
| C | ��ZnS����ˮ���γɰ�ɫ����Һ���������м���CuSO4��Һ | �к�ɫ�������� | Ksp��ZnS����Ksp��CuS�� |
| D | ���������������Ũ������ | ���������� | Ũ�������ǿ������ |
| A�� | A | B�� | B | C�� | C | D�� | D |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 28g���������е�ԭ����ĿΪNA | |
| B�� | �ڳ��³�ѹ�£�11.2L N2���еķ�����Ϊ0.5NA | |
| C�� | 0.5mol���������������ᷴӦת�Ƶ�����Ϊ1.5NA | |
| D�� | ��״���£�1Lˮ����������Ϊ1/22.4NA |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����

| A�� | ȡ�����M���A��B���ּ�Һ����ͬŨ�ȵ�������Һ��ǡ����ȫ��Ӧʱ������������Һ�������ͬ | |
| B�� | �ô����к�A��Һ��ǡ����ȫ��Ӧʱ����Һ��pH��һ������7 | |
| C�� | ϡ��ǰ����Һ��H+Ũ�ȵĴ�С��ϵ��A=10B | |
| D�� | ϡ��ǰ��A��Һ����ˮ�������OH-��Ũ�ȴ���10-7mol/L |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ������ | B�� | ����������Һ | C�� | ̼������Һ | D�� | ���Ƶ�������ͭ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
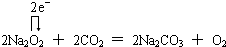 ��
���鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com