
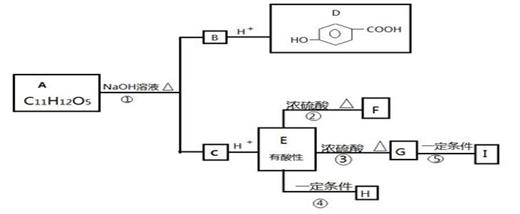
ij�����廯����A��ˮ��Һ�����ԣ����A���������ǻ���A�ɷ�����ͼ��ʾת��������FΪ��Ԫ��״�����G��ʹ������Ȼ�̼��Һ��ɫ��F��G��Ϊͬ���칹�壻H��I����ҽ�ø߷��Ӳ��ϡ�
��ش�
��1����Ӧ�ڵĻ�ѧ����ʽ�� ��
��2����2��A��H�Ľṹ��ʽ�ֱ��� �� ��
��3��D������X��Һ��Ӧ��ɵõ�C7H5O3Na����X��Һ���������ʵĻ�ѧʽ�� ��
��4����������������G��ͬ���칹���еķ�ʽ�ṹ�Ľṹ��ʽ
��
���ܷ���ˮ�ⷴӦ �ڷ����в�����״�ṹ �۷�������4�ֲ�ͬ��ѧ��������ԭ�ӣ�
 Ӧ�����������Ĵ���ѧ������ϵ�д�
Ӧ�����������Ĵ���ѧ������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���л�������ͬʱ�����Ӽ������Լ����Ǽ��Լ����ǣ� ��
A��MgCl2 B��H2O2
C��NaOH D��CH3COONH4
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��2L�����ܱ������г���2 mol X��1mol Y������Ӧ��2X(g)+Y(g) 3Z(g) ��H<0����Ӧ���̳��������¶ȣ���û����ϵ��X������������¶ȵĹ�ϵ��ͼ��ʾ�������ƶ���ȷ����
3Z(g) ��H<0����Ӧ���̳��������¶ȣ���û����ϵ��X������������¶ȵĹ�ϵ��ͼ��ʾ�������ƶ���ȷ����
A�������¶ȣ�ƽ�ⳣ������
B��W��X������Ӧ���ʵ���M��X������Ӧ����
C��Q��ʱ��Y��ת�������
D��ƽ��ʱ����Z���ﵽ��ƽ��ʱZ�����������ԭƽ��ʱ��

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����ʵ���У�Ϊʵ��ʵ��Ŀ�ı�����ӣ�������ȷ���� �� ��
A���٢ڢۢܢ� B��ֻ�Тڢܢ� C��ֻ�Тڢۢ� D��ֻ�Т٢ڢܢ�
| ʵ�� | �����Լ� | ʵ��Ŀ�� | |
| �� | ��ʯ��ˮ��Ӧ | CuSO4��Һ | ��KMnO4������Һ������Ȳ�Ļ�ԭ�� |
| �� | CH3CH2Br��NaOH��Һ���� | HNO3��Һ | ��AgNO3��Һ����CH3CH2 Br�е�Br |
| �� | ������ϡH2 SO4ˮԡ���� | HNO3��Һ | ��������Һ����ˮ�����Ļ�ԭ�� |
| �� | C2H5OH��ŨH2SO4������170�� | NaOH��Һ | ��KMnO4��Һ֤���÷�ӦΪ��ȥ��Ӧ |
| �� | ����Һ�巴Ӧ | CC14 | ��AgNO3��Һ֤���÷�ӦΪȡ����Ӧ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�¡�������ͪ������õ�廨�����ѵ��е�һ����Ȼ���ϣ������ಽ��Ӧ�ɺϳ�ά����A1��
����˵����ȷ���� �� ��
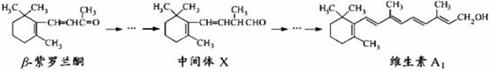
A.��������KMnO4��Һ�����м���X�д���̼̼˫��
B.�¡�������ͪ��H2��ּӳɺ��к�3������̼
C.ά����A1������ˮ������������֬
D.�¡�������ͪ���м���X��Ϊͬϵ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����SO2�������Ƿ����CO2���壬�ɲ��õķ�����(����)
A��ͨ������ʯ��ˮ
B����ͨ������NaHCO3��Һ����ͨ������ʯ��ˮ
C����ͨ��NaOH��Һ����ͨ������ʯ��ˮ
D����ͨ������KMnO4��Һ����ͨ��Ʒ����Һ�����ͨ������ʯ��ˮ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ҵ��������ɰ��SiC���Ļ�ѧ����ʽ���£�SiO2+3C SiC+2CO���������������ԭ��Ӧ�У��������뻹ԭ�����ʵ���֮����
SiC+2CO���������������ԭ��Ӧ�У��������뻹ԭ�����ʵ���֮����
A��2 ��1 B��1 ��2 C��5 ��3 D��3 ��5
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���з�Ӧ�����ӷ���ʽ��ȷ����
A��Na2CO3ˮ�⣺CO32��+H2O��H2CO3+2OH��
B��AgCl���ڰ�ˮ��AgCl+2NH3��H2O��[Ag(NH3)2]++Cl��+2H2O
C����NaAlO2��Һ��ͨ�������CO2��2AlO +CO2+3H2O��2Al(OH)3��+CO32��
+CO2+3H2O��2Al(OH)3��+CO32��
D��������KMnO4��Һ��ͨ��SO2��2MnO4��+5SO2+4OH����2Mn2++5SO42��+2H2O
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com