
�����������������ƾ���Ϊ����ȼ�ϵļ����Ѿ�����ȫ���ƹ㡣��֪C8H18��C2H5OHȼ�յ��Ȼ�ѧ����ʽ�ֱ�Ϊ��
2C8H18(l)��25O2(g)===16CO2(g)��18H2O(l)����H����10900 kJ·mol��1
C2H5OH(l)��3O2(g)===2CO2(g)��3H2O(l)�� ��H����1367 kJ·mol��1
�ٶ����͵ijɷ�ΪC8H18���������Ӿƾ�������������ȼ��ʱ�����ܴﵽ��Ŀ����(����)
A����ʡ��ʯȼ��
B�������к�������ŷ�
C��������ת����ʣ����ʳ
D�����ÿǧ��ȼ��ȼ�շų�������
�𰸡�D
���������������������Ҵ�ȼ�շų������������Լ��������͵����ģ��������ǻ�ʯȼ�ϣ�A����ȷ�������Ҵ����������Դ��������ȼ�յ���ȫ�����������Ҵ���ȼ�Ͽɼ����к�������ŷţ�B����ȷ���Ҵ���ͨ����ʳ�ķ�������ȡ��(C6H10O5)n��nH2O����nC6H12O6��C6H12O6����2CH3CH2OH��2CO2�������������ù�ʣ����ʳ��ͬʱ�ֲ���Դ�IJ��㣬C����ȷ�����Ȼ�ѧ����ʽ֪��ȼ��1 g C8H18�ų�������Ϊ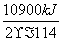 ��48 kJ��ȼ��1 g�Ҵ��ų�������Ϊ
��48 kJ��ȼ��1 g�Ҵ��ų�������Ϊ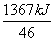 ��30 kJ�������������Ӿƾ���ÿǧ��ȼ��ȼ�շų������������½���D�����
��30 kJ�������������Ӿƾ���ÿǧ��ȼ��ȼ�շų������������½���D�����


 �п������п��Ծ����ϵ�д�
�п������п��Ծ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����������ʢ�й���ϡ������Թ��У���Ӱ�������������ʵ�������(����)
A�������Ũ�� B�������ı����
C����Ӧ���¶� D��������Na2SO4����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ͼ�ǵ��CuCl2��Һ��װ�ã�����c��dΪʯī�缫���������йص��ж���ȷ����(����)

A��a������b����
B��a������b����
C���������У�d�缫��������
D���������У�������Ũ�Ȳ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����Ȼ�ѧ����ʽ�Ц�H����ȼ���ȵ���(����)
A��CH4(g)��3/2O2(g)===2H2O(l)��CO(g)����H1
B��S(s)��3/2O2(g)===SO3(g)����H2
C��C6H12O6(s)��6O2(g)===6CO2(g)��6H2O(l)����H3
D��2CO(g)��O2(g)===2CO2(g)����H4
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�������������Ȼ�ѧ����ʽ��
(1)H2(g)�� O2(g)===H2O(g)����H1��a kJ·mol��1��
O2(g)===H2O(g)����H1��a kJ·mol��1��
(2)H2(g)�� O2(g)===H2O(l)����H2��b kJ·mol��1
O2(g)===H2O(l)����H2��b kJ·mol��1
(3)2H2(g)��O2(g)===2H2O(l)����H3��c kJ·mol��1
���й������ǵ�������ȷ����(����)
A�����Ƕ������ȷ�Ӧ
B��a��b��c��Ϊ��ֵ
C��a��b
D��2b��c>0
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����������ȼ�գ������������ֹ�̬������(P2O3��P2O5)��3.1 g�ĵ�����(P)��3.2 g������ȼ�գ�����Ӧ��ľ����ų�x kJ������
(1)ͨ������ȷ����Ӧ��������(�û�ѧʽ��ʾ)��_________��
����Ӧ������(g)�ֱ�Ϊ______________��
(2)��֪������ȼ����Ϊy kJ·mol��1����ʾ������ȼ�յ��Ȼ�ѧ����ʽΪ______________________________��
(3)����P2O3�������з�Ӧ���ɹ���P2O5���Ȼ�ѧ����ʽΪ________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����˵���У���ȷ����(����)
A������������ָ���ͱ���ͬϵ��
B��ͨ���ɴ�ú�����л�ʯ�͵Ĵ���������ȡ������
C���ұ�����������ԭ�ӿ��Դ���ͬһƽ����
D�����ͼױ�ֻ�ܷ���ȡ����Ӧ���ܷ���������Ӧ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����˵����ȷ���� �� ��
A��1 mLŨ��ˮ��ˮϡ����l00mL����Һ��n��OH-������
B��NaHCO3��ȫ�ֽ��Ĺ�������ˮ��������Һ�в�����HCO3-
C�������£�pH=3�Ĵ����pH=11��NaOH��Һ�������Ϻ���Һ��pH<7
D�������£���NH4Cl��Һ�м��백ˮ����Һ��pH=7����ʱ��Һ��c��NH4+��>c��C1-��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com