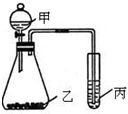
ĻĀ±ķĪŖ³¤Ź½ÖÜĘŚ±ķµÄŅ»²æ·Ö£¬ĘäÖŠµÄ±ąŗÅ“ś±ķ¶ŌÓ¦µÄŌŖĖŲ£®
| ¢Ł |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
¢Ś |
|
|
|
|
|
|
|
|
|
|
|
¢Ū |
¢Ü |
¢Ż |
¢Ž |
|
|
¢ß |
|
|
|
|
|
|
|
|
|
|
|
|
|
¢ą |
|
|
|
|
|
|
|
¢į |
|
|
|
|
¢ā |
|
|
|
|
|
|
|
£Ø1£©Š“³öÉĻ±ķÖŠŌŖĖŲ¢įŌ×ÓµÄĶāĪ§µē×ÓÅŲ¼Ź½
3d54s1
3d54s1
£®
£Ø2£©ŌŚŌŖĖŲ¢ŪÓė¢ŁŠĪ³ÉµÄĖ®¹ū“ߏģ¼ĮĘųĢå»ÆŗĻĪļÖŠ£¬ŌŖĖŲ¢ŪµÄŌӻƷ½Ź½ĪŖ£ŗ
sp2
sp2
£Ø3£©°“ŅŖĒóĶź³ÉĻĀĮŠø÷Ģā
a£®µŚŅ»µēĄėÄÜ£ŗŌŖĖŲ¢Ü
£¾
£¾
ŌŖĖŲ¢Ż£ØŃ”Ģī”°£¾”±”¢”°=”±”¢”°£¼”±£©£®
b£®ÓėŌŖĖŲ¢ÜĖłŠĪ³ÉµÄµ„ÖŹ»„ĪŖµČµē×ÓĢåµÄ·Ö×Ó”¢Ąė×ӵĻÆѧŹ½
CO
CO
Ӣ
C22-
C22-
£Øø÷Š“Ņ»ÖÖ£©£®
c£®ŌŖĖŲ¢ÜµÄĘųĢ¬Ēā»ÆĪļXµÄĖ®ČÜŅŗŌŚĪ¢µē×Ó¹¤ŅµÖŠ£¬æÉ×÷æĢŹ“¼ĮH
2O
2µÄĒå³ż¼Į£¬Ėł·¢Éś·“Ó¦µÄ²śĪļ²»ĪŪČ¾»·¾³£¬Ęä»Æѧ·½³ĢŹ½ĪŖ
2NH3£®H2O+3H2O2ØTN2+8H2O
2NH3£®H2O+3H2O2ØTN2+8H2O
d£®ÓÉXÓėŃõĘų”¢KOHČÜŅŗ¹¹³ÉŌµē³Ų£¬øŗ¼«»į²śÉśŌŖĖŲ¢ÜµÄµ„ÖŹ£®ŌņĘäøŗ¼«·“Ó¦Ź½ĪŖ
2NH3+6OH--6e-ØTN2+6H2O
2NH3+6OH--6e-ØTN2+6H2O
£®
£Ø4£©ÓÉŌŖĖŲ¢ŪŗĶ¢ąŠĪ³ÉµÄŅŗĢ¬»ÆŗĻĪļZ£¬ŹĒ·Ē¼«ŠŌµÄÖ±ĻߊĪ·Ö×Ó£®0.2molµÄZŌŚO
2ÖŠĶźČ«Č¼ÉÕ£¬Éś³ÉĮ½ÖÖĘųĢ¬Ńõ»ÆĪļ£¬298KŹ±·Å³öČČĮæ215kJ£®øĆ·“Ó¦µÄČČ»Æѧ·½³ĢŹ½ĪŖ
CS2£Øl£©+3O2£Øg£©ØTCO2£Øg£©+2SO2£Øg£©”÷H=-1075kJ/mol
CS2£Øl£©+3O2£Øg£©ØTCO2£Øg£©+2SO2£Øg£©”÷H=-1075kJ/mol
£Ø5£©ŌŚ²ā¶Ø¢ŁÓė¢ŽŠĪ³É»ÆŗĻĪļµÄĻą¶Ō·Ö×ÓÖŹĮæŹ±£¬ŹµŃé²āµĆµÄÖµŅ»°ćøßÓŚĄķĀŪÖµµÄÖ÷ŅŖŌŅņŹĒ£ŗ
·Ö×ÓÖ®¼äŠĪ³ÉĒā¼ü
·Ö×ÓÖ®¼äŠĪ³ÉĒā¼ü
£®
£Ø6£©ŌŖĖŲ¢āĖłŠĪ³ÉµÄµ„ÖŹ¾§ĢåÖŠŌ×ӵĶѻż·½Ź½ČēĻĀĶ¼¼×ĖłŹ¾£¬Ę侧°ūĢŲÕ÷ČēĶ¼ŅŅĖłŹ¾£¬Ō×ÓÖ®¼äĻą»„Ī»ÖĆ¹ŲĻµµÄĘ½ĆęĶ¼ČēĶ¼±ūĖłŹ¾£® ŅŃÖŖøĆŌ×ӵİė¾¶ĪŖd£¬Ļą¶ŌŌ×ÓÖŹĮæĪŖM£¬N
A“ś±ķ°¢·ü¼ÓµĀĀŽ³£Źż£¬Ēė»Ų“š£ŗ

¾§ĢåÖŠøĆŌ×ÓµÄÅäĪ»ŹżĪŖ
12
12
£¬Ņ»øö¾§°ūÖŠ°üŗ¬µÄŌ×ÓŹżÄæĪŖ
4
4
£»øĆ¾§ĢåµÄĆܶČĪŖ
£ØÓĆ×ÖÄø±ķŹ¾£©

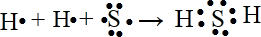
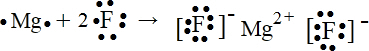
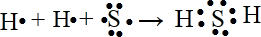
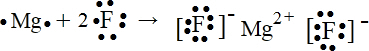












 µÄ·Ö×ÓŹ½ŹĒ
µÄ·Ö×ÓŹ½ŹĒ ÓŠ»śĪļYŹĒXµÄĶ¬·ÖŅģ¹¹Ģ壬ĒŅŹōÓŚ·¼ĻćĢž£¬Š“³öYµÄ½į¹¹¼ņŹ½
ÓŠ»śĪļYŹĒXµÄĶ¬·ÖŅģ¹¹Ģ壬ĒŅŹōÓŚ·¼ĻćĢž£¬Š“³öYµÄ½į¹¹¼ņŹ½
