
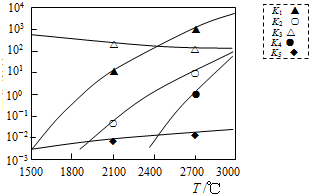
���� ��1�����¶�Խ��KֵԽС�ķ�Ӧ�Ƿ��ȷ�Ӧ����Ӧ������С����ƽ�ⳣ��������ƽ̹���ɴ˷������
���ɷ�Ӧ����CaO��s��+C��s��?Ca��g��+CO��g����H1=a kJ•mol-1����Ca��g��+2C��s��?CaC2��s����H2=b kJ•mol-1�����ݸ�˹���ɣ�Ŀ�귴Ӧ�ķ�Ӧ��Ϊ���ڡ�2-�۵ã�
��2����������������ɢ������ת���ʾ�Ϊ100%������¯�г���������ֻ��CO����Ϊ��ά����ƽ�⣬����ÿ����1molCaC2����Ͷ�ϵ���Ϊ��1molCaO����Ͷ��̼����Ϊ��3mol+$\frac{464.1kJ}{110.5kJ•mo{l}^{-1}}$=7.2mol�����������ʵ���Ϊ��$\frac{464.1kJ}{110.5kJ•mo{l}^{-1}}$��$\frac{1}{2}$=2.1mol��
��3������Ȳ��Ĺ��������Ҫ�ɷ�ΪCa��OH��2����������ȡƯ�ۣ�����ȡƯ�۵Ļ�ѧ����ʽΪ2Ca��OH��2+2Cl2=CaCl2+Ca��ClO��2+2H2O������Ȳ����������֮һPH3������������ȩ��������70�桢Al��OH��3���������£��ɺϳ�THPC��ȼ��{[P��CH2OH��4]Cl }���÷�Ӧ�Ļ�ѧ����ʽΪPH3+4 HCHO+HCl $\frac{\underline{\;��������\;}}{70��}$[P��CH2OH��4]Cl��
��� �⣺��1�����¶�Խ��KֵԽС�������Ǣۣ����Ԣ��Ƿ��ȷ�Ӧ����Ӧ������С����ƽ�ⳣ��������ƽ̹�Ǣݣ��ʴ�Ϊ���ۣ��ݣ���2�֣���
���ɷ�Ӧ����CaO��s��+C��s��?Ca��g��+CO��g����H1=a kJ•mol-1����Ca��g��+2C��s��?CaC2��s����H2=b kJ•mol-1�����ݸ�˹���ɣ�Ŀ�귴Ӧ�ķ�Ӧ��Ϊ���ڡ�2-�۵á�H3=��2a-b��kJ•mol-1���ʴ�Ϊ��2a-b��
��2����������������ɢ������ת���ʾ�Ϊ100%������¯�г���������ֻ��CO����Ϊ��ά����ƽ�⣬����ÿ����1molCaC2����Ͷ�ϵ���Ϊ��1molCaO����Ͷ��̼����Ϊ��3mol+$\frac{464.1kJ}{110.5kJ•mo{l}^{-1}}$=7.2mol�����������ʵ���Ϊ��$\frac{464.1kJ}{110.5kJ•mo{l}^{-1}}$��$\frac{1}{2}$=2.1mol���ʴ�Ϊ��7.2��2.1��
��3������Ȳ��Ĺ��������Ҫ�ɷ�ΪCa��OH��2����������ȡƯ�ۣ�����ȡƯ�۵Ļ�ѧ����ʽΪ2Ca��OH��2+2Cl2=CaCl2+Ca��ClO��2+2H2O������Ȳ����������֮һPH3������������ȩ��������70�桢Al��OH��3���������£��ɺϳ�THPC��ȼ��{[P��CH2OH��4]Cl }���÷�Ӧ�Ļ�ѧ����ʽΪPH3+4 HCHO+HCl $\frac{\underline{\;��������\;}}{70��}$[P��CH2OH��4]Cl���ʴ�Ϊ��2Ca��OH��2+2Cl2=CaCl2+Ca��ClO��2+2H2O��PH3+4 HCHO+HCl $\frac{\underline{\;��������\;}}{70��}$[P��CH2OH��4]Cl��
���� ���⿼��ѧ����ѧƽ�ⳣ������˹�����ԡ���ѧƽ���ƶ���Ӱ��ͻ�ѧ����ʽ����д�����֪ʶ���ۺ���ǿ���ѶȲ���


 ���Ž�������С״Ԫϵ�д�
���Ž�������С״Ԫϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ����ɫ��Һ�У�NH4+��Fe2+��SO42-��CO32- | |
| B�� | �ں�����Ba2+��Һ�У�NH4+��Na+��Cl-��OH- | |
| C�� | ��ǿ����Һ�У�Na+��K+��Cl-��SO32- | |
| D�� | �����Ե���Һ�У�K+��Fe2+��Cl-��CH3COO- |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��ԭ���� | B�� | ���� | C�� | ԭ���� | D�� | ��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
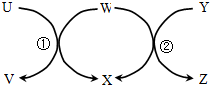 ��ͼ��U��Z�����������ʵ���������ʵ��ͼʾ��ͷ����һ��ת�����ҷ�Ӧ�١��ھ�Ϊ�û���Ӧ�������������������ǣ�������
��ͼ��U��Z�����������ʵ���������ʵ��ͼʾ��ͷ����һ��ת�����ҷ�Ӧ�١��ھ�Ϊ�û���Ӧ�������������������ǣ�������| ��� | U | W | Y | X |
| �� | Na | H2O | Na2O2 | NaOH |
| �� | Fe | H2O | C | H2 |
| �� | HBr | Cl2 | CH4 | HCl |
| �� | CuCl2��aq�� | Al | HCl��aq�� | AlCl3��aq�� |
| A�� | �ڢ� | B�� | �ڢ� | C�� | �٢ڢ� | D�� | �٢ڢۢ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 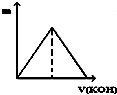 | B�� | 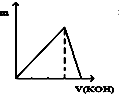 | C�� | 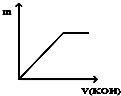 | D�� | 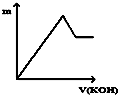 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
| ���ܵ���� | Cu��OH��2 | CuS | Pb��OH��2 | PbS |
| Ksp | 4.8��10-20 | 6.3��10-36 | 1.2��10-15 | 1.0��10-28 |
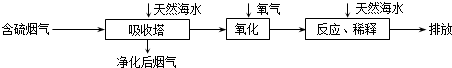

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �ȵĴ�����Һ����Ĵ�����Һȥ����Ч���� | |
| B�� | ʢ��Na2CO3��Һ���Լ�ƿ���������� | |
| C�� | ��AlCl3��Һ���ȡ����ɡ����գ��ɵõ�����Al2O3 | |
| D�� | ���ڳ�ʪ�Ļ���������������ˮ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �Ʊ�����ϸ��ƿ�в���ú��Һ�� | |
| B�� | ������ˮ��������ɫƿ�в������䰵�� | |
| C�� | NaOH��Һ��������ɫ�Լ�ƿ�в�Ҫ�������� | |
| D�� | ����������Һ����ڼ����������۵��Լ�ƿ�� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com