
���ӷ�Ӧ����ѧ��ѧ����Ҫ�ķ�Ӧ���͡��ش��������⣺
(1)�ڷ������ӷ�Ӧ�ķ�Ӧ����������У�һ�������� (�����)��
�ٵ��ʣ���������۵���ʣ����Σ��ݻ�����
(2)����ͼʾ�ķ�����ʾ��ͬ��Ӧ����֮��Ĺ�ϵ����ֽⷴӦ��������ԭ��Ӧ�ɱ�ʾΪ��ͼ����������ķ����л������ӷ�Ӧ���û���Ӧ��������ԭ��Ӧ����֮��Ĺ�ϵ��

(3)���ӷ���ʽ����Ҫ�Ļ�ѧ����������й����ӷ���ʽ��һЩ����۵㣬�������б���������Ӧ�ġ����ӷ���ʽ������Щ�۵㡣
| �����е����ӷ���ʽ�����Ա�ʾһ�෴Ӧ | |
| ������кͷ�Ӧ���ɱ�ʾΪH����OH��=H2O | |
| �����ӷ���ʽ�з����������ᡢ��ξ�Ҫ�ꡰ�������� | |

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
���л�ѧ��Ӧ�����ӷ���ʽ����ȷ����
| A��Na2S��Һ�м�������FeCl3��Һ��2Fe3+��S2�� �� 2Fe2+��S�� |
| B����NaOH��Һ�еμ�̼�������Һ��OH��ǡ����ȫ��Ӧ��Ca2++2OH����2HCO3�� �� CaCO3����2H2O��CO32�� |
| C����NaClO��Һ��ͨ������SO2���壺ClO����SO2��H2O �� SO42����Cl����2H+ |
D��Na2CO3��Һ�е����������ǻ���������Һ��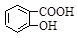 ��CO32�� ��CO32�� 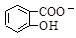 ��HCO3�� ��HCO3�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��15�֣�ʵ��̽����ѧϰ��ѧ��һ����Ҫ������ijʵ��С���ͬѧ��������װ�����һЩ���������Ʊ��Լ������������̽�����г�װ�ü���������ʡ�ԣ�����װ��E�ж����ʹ�ã���
�ɹ�ѡ���Һ���Լ�������ҩƷ��
| Һ���Լ� | ����ҩƷ |
| ϡ���ᡢϡ���ᡢϡ���ᡢNaOH��Һ��Ũ��ˮ��5%H2O2��Һ��Ũ���ᡢ����ʳ��ˮ | CaCO3��CaO��MnO2��KMnO4��CaC2�� ��ʯ�ҡ�Cu��Zn��Na2S |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��Ƴ���ͭ��ˮ�к���CN-��Cr2O72-����,��Ҫ������������ŷš��ó��ⶨ�������̽��з�ˮ������ �ش��������⣺
��1������������ˮ��������Ҫʹ�õķ����� ��
��2�����з�Ӧ��������ų����÷�Ӧ�����ӷ���ʽΪ ��
��3��������У�ÿ����0.4mol Cr2O72-ʱת�Ƶ���2.4mol���÷�Ӧ���ӷ���ʽΪ ��
��4��ȡ��������ˮ�����Թ��У�����NaOH��Һ���۲쵽����ɫ�������ɣ��ټ�Na2S��Һ�� ��ɫ����ת���ɺ�ɫ��������ʹ�û�ѧ��������ֽ��Ͳ����������ԭ�� ��
��5��Ŀǰ��������Cr2O72-��ˮ������������巨���÷������ˮ�м���FeSO4��7H2O��Cr2O72-��ԭ��Cr3+������pH��Fe��Crת�����൱�� (��������,�������ֱ�ʾԪ�ؼ�̬)�ij���������1mol Cr2O72-�������a mol FeSO4?7H2O�����н�����ȷ���� ��
(��������,�������ֱ�ʾԪ�ؼ�̬)�ij���������1mol Cr2O72-�������a mol FeSO4?7H2O�����н�����ȷ���� ��
| A��x ="0.5" ,a =8 | B��x ="0.5" ,a = 10 | C��x =" 1.5" ,a =8 | D��x =" 1.5" ,a = 10 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
����A��B��C��D��E���ֻ������֪���ǵ���������Al3+��Fe2+��K+��Ba2+��Ag+����������SO42-��Cl-��CO32-��NO3-��OH-�ֽ����Ƿֱ����0��1mol/L����Һ��������ʵ��:
�ٲ��C��E��Һ�Լ��ԣ��Ҽ���E��C
��A��C��Һ��Ϻ��а�ɫ��������ɫ��ζ���������ɣ���������E��Һ����Һ�����
��A��Һ�м���D��Һ��Ҳ���ְ�ɫ�������ó���������ϡ����
��B��Һ�м���E��Һ���ְ�ɫ����������Ѹ�ٱ�ɻ���ɫ������ɺ��ɫ
��������ʵ�飬�ش��������⣺
��1�� д���������ʵĻ�ѧʽ
A �� B �� C �� D �� E ��
��2����Ҫ��д�����з�Ӧ�ķ�Ӧ����ʽ��
A��C ��Ӧ�����ӷ���ʽ�� ��
A�м��������E��Һ������ӷ���ʽ ��
B��E��Ӧ��İ�ɫ�����ڿ��������ձ�ɺ��ɫ�Ļ�ѧ����ʽ�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�������������������м�����Ҫ�����ã��������й��ա���Ч���Ͷ���ɱ�������������ǽ��չ�����ơ�
(1)Cl2��H2O2��ClO2(��ԭ����ΪCl��)��O3(1 mol O3ת��Ϊ1 mol O2��1 mol H2O)�����ʳ��������������������ʵ�����������������Ч����ߵ���________(�����)��
| A��Cl2 | B��H2O2 | C��ClO2 | D��O3 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��֪����ǿ�������Һ���ֱ������������������еĸ�һ�֣����һ����ظ���NH4����Ba2����Na����H����SO42����NO3����OH����SO32��������������Һ�ֱ���ΪA��B��C��D����������ʵ�顣
����A��D�е���C�����г������ɣ�
��D��B��Ӧ���ɵ������ܱ�A���գ�
��A��D��Ӧ���ɵ������ܱ�B���գ�Ҳ��ʹ��ˮ��ɫ��
�Իش��������⣺
(1)D�Ļ�ѧʽ��________���ж�������___________________________________________��
(2)д�����༸�����ʵĻ�ѧʽ��A________��B________��C________��
(3)д��ʵ������йط�Ӧ�����ӷ���ʽ_______________________________��
(4)д���������ɵ���������ˮ��Ӧ�����ӷ���ʽ_______________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
ij�����Һ�п��ܺ��е��������±���ʾ:
| ���ܴ������е������� | H+��Ag+��Mg2+��Al3+��NH4+��Fe3+ |
| ���ܴ������е������� | Cl-��Br-��I-��CO32����AlO2�� |
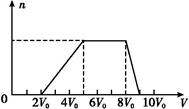
| Cl2�����(��״��) | 11.2 L | 22.4 L | 28.0 L |
| n(Cl-) | 2.5 mol | 3.5 mol | 4.0 mol |
| n(Br-) | 3.0 mol | 2.5 mol | 2.0 mol |
| n(I-) | x mol | 0 | 0 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
ij��ɫ��Һֻ���ܺ������¼������ӣ���Mg2������Al3������Fe2������H������ ����Cl������OH���������л����ص���NaOH��Һ���������������������������NaOH��Һ������Ĺ�ϵ��ͼ��ʾ���ɴ˿�ȷ��ԭ��Һ��һ�����е�������
����Cl������OH���������л����ص���NaOH��Һ���������������������������NaOH��Һ������Ĺ�ϵ��ͼ��ʾ���ɴ˿�ȷ��ԭ��Һ��һ�����е�������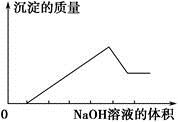
| A���٢ڢ� | B���٢ۢ� | C���ڢޢ� | D���٢ڢܢ� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com