
�������ͼ����
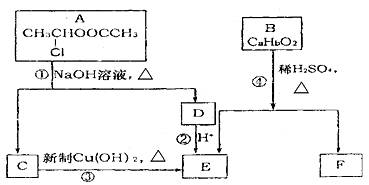
��֪��һ��̼ԭ�������������ǻ�ʱ����������ת����
![]()
��1��E�к��еĹ������� ��
��2����Ӧ�۵Ļ�ѧ����ʽ�� ��
��3����֪B����Է�������Ϊ162������ȫȼ�յIJ�����n��CO2����n��H2O��=2��1����B�ķ���ʽΪ ��
��4��F�Ǹ߷��ӹ������������Ҫԭ�ϡ�F���������ص㣺
���ܸ�FeC13��Һ������ɫ��Ӧ��
���ܷ����Ӿ۷�Ӧ��
�۱����ϵ�һ�ȴ���ֻ�����֡�
F��һ�������·����Ӿ۷�Ӧ�Ļ�ѧ����ʽΪ ��
��5��������G��F��ͬ���칹�壬���ڷ����廯����ܷ���������Ӧ��G�ж��ֽṹ��д������һ�ֵĽṹ��ʽ ��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
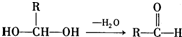
| �� |
| �� |
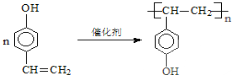

 ������ˮ����ᆳ�ۺϷ�Ӧ��ɵõ��߾��CaHbO2��nH�ж��ֽṹ��д������һ�ֵĽṹ��ʽ
������ˮ����ᆳ�ۺϷ�Ӧ��ɵõ��߾��CaHbO2��nH�ж��ֽṹ��д������һ�ֵĽṹ��ʽ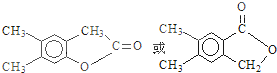

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
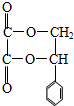



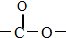 �����ţ�������Ҳ�����������Ӧ��д������һ��1mol�������������3molNaOH��ͬ���칹��Ľṹ��ʽ
�����ţ�������Ҳ�����������Ӧ��д������һ��1mol�������������3molNaOH��ͬ���칹��Ľṹ��ʽ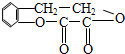
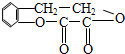
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

| �� |
| �� |
| �� |
| �� |


 ��
�� ��
�� ��
�� ����2��
����2�� ��
�� ��
�� ��
�� ����2��
����2���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
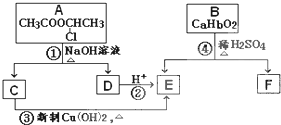
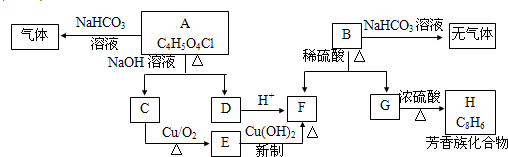
(��15��) �������ͼ����
��֪��һ��̼ԭ�������������ǻ�ʱ����������ת����
��1����Ӧ���������л���Ӧ������_______________��Ӧ��
��2����Ӧ�۵Ļ�ѧ����ʽ______________________________________________________��
��3����֪B����Է�������Ϊ162������ȫȼ�յIJ�����n(CO2)��n (H2O) =2��1����B�ķ���ʽΪ ��
��4��F�Ǹ߷��ӹ���������е���Ҫԭ�ϡ�F���������ص㣺���ܸ�FeCl3��Һ������ɫ��Ӧ�����ܷ����Ӿ۷�Ӧ���۱����ϵ�һ�ȴ���ֻ�����֡�F��һ�������·����Ӿ۷�Ӧ�Ļ�ѧ����ʽΪ ��
��5��������G��F��ͬ���칹�壬���ڷ����廯����ܷ���������Ӧ��G�Ľṹ�� �֡�
��6��������H��B��ͬ���칹�壬H�����к��еIJ��ֽṹΪ������ˮ����ᆳ�ۺϷ�Ӧ��ɵõ��߾���(CaHbO2)n.��H�ж��ֽṹ��д������һ�ֵĽṹ��ʽ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010�꺣��ʡ������У������ѧ���� ���ͣ������
���л���ѧ������
18-1����6�֣�����Ϊ����ѡ���⣬ȫѡ�Ե�6�֣�©ѡ���������֣���ѡ���ѡΪ0�֣�
���й����л����˵���У���ȷ���ǣ� ��
| A�������Ǹ߷��ӻ������ˮ������ܷ���������Ӧ |
| B������ά��һ�����Ǻϳɸ߷��Ӳ��� |
| C�������µ�������Ʊ���ɫ����������������Cu��OH��2������Ӧ |
| D����ij����Ļ�������Cl2��Ϲ��գ���������״Һ�����ɣ�˵���������϶����м��� |
 ����������ԭ�Ӳ����ܹ�ƽ��
����������ԭ�Ӳ����ܹ�ƽ��

�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com