
ŠæŹĒŅ»ÖÖÓ¦ÓĆ¹ć·ŗµÄ½šŹō£¬ÄæĒ°¹¤ŅµÉĻÖ÷ŅŖ²ÉÓĆ”°ŹŖ·Ø”±¹¤ŅÕŅ±Į¶Šæ”£Ä³ŗ¬ŠææóµÄÖ÷ŅŖ³É·ÖĪŖZnS£Ø»¹ŗ¬ÉŁĮæFeSµČĘäĖū³É·Ö£©£¬ŅŌĘäĪŖŌĮĻŅ±Į¶ŠæµÄ¹¤ŅÕĮ÷³ĢČēĶ¼ĖłŹ¾£ŗ
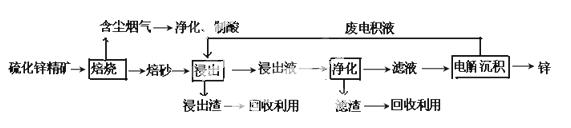
»Ų“šĻĀĮŠĪŹĢā£ŗ
£Ø1£©Įņ»ÆŠæ¾«æóµÄ±ŗÉÕŌŚŃõĘųĘų·ÕµÄ·ŠĢŚĀÆÖŠ½ųŠŠ£¬Ėł²śÉś±ŗÉ°µÄÖ÷ŅŖ³É·ÖµÄ»ÆѧŹ½ĪŖ____”£
£Ø2£©±ŗÉÕ¹ż³ĢÖŠ²śÉśµÄŗ¬³¾ŃĢĘųæɾ»»ÆÖĘĖį£¬øĆĖįæÉÓĆÓŚŗóŠųµÄ_______²Ł×÷.
£Ø3£©½ž³öŅŗ”°¾»»Æ”±¹ż³ĢÖŠ¼ÓČėµÄÖ÷ŅŖĪļÖŹĪŖ________£¬Ęä×÷ÓĆŹĒ__________________”£
£Ø4£©µē½ā³Į»ż¹ż³ĢÖŠµÄŅõ¼«²ÉÓĆĀĮ°å£¬Ńō¼«²ÉÓĆPb-AgŗĻ½š¶čŠŌµē¼«£¬Ńō¼«ŅŻ³öµÄĘųĢåŹĒ____”£
£Ø5£©øĽųµÄŠæŅ±Į¶¹¤ŅÕ£¬²ÉÓĆĮĖ”°ŃõŃ¹Ėį½ž”±µÄČ«ŹŖ·ØĮ÷³Ģ£¬¼ČŹ”ĀŌĮĖŅ×µ¼ÖĀæÕĘųĪŪČ¾µÄ±ŗÉÕ¹ż³Ģ£¬ÓÖæÉ»ńµĆŅ»ÖÖÓŠ¹¤Ņµ¼ŪÖµµÄ·Ē½šŹōµ„ÖŹ”£”°ŃõŃ¹Ėį½ž”±ÖŠ·¢ÉśµÄÖ÷ŅŖ·“Ó¦µÄĄė×Ó·½³ĢŹ½ĪŖ___________________”£
£Ø6£©ĪŅ¹ś¹Å“śŌų²ÉÓĆ”°»š·Ø”±¹¤ŅÕŅ±Į¶Šæ”£Ć÷“śĖĪÓ¦ŠĒÖųµÄ”¶Ģģ¹¤æŖĪļ”·ÖŠÓŠ¹ŲÓŚ ”°ÉżĮ¶ŁĮĒ¦”±µÄ¼ĒŌŲ£ŗ”°ĀÆøŹŹÆŹ®½ļ£¬×°ŌŲČėŅ»Äą¹ŽÄŚ£¬””£¬Č»ŗóÖš²ćÓĆĆŗ Ģæ±żµęŹ¢£¬Ęäµ×ĘĢŠ½£¬·¢»šģŃŗģ£¬””£¬Ąäµķ£¬»Ł¹ŽČ”³ö£¬””£¬¼“
Ģæ±żµęŹ¢£¬Ęäµ×ĘĢŠ½£¬·¢»šģŃŗģ£¬””£¬Ąäµķ£¬»Ł¹ŽČ”³ö£¬””£¬¼“ ŁĮĒ¦Ņ²”£”±øĆĮ¶Šæ¹¤ŅÕ¹ż³ĢÖ÷ŅŖ·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ____”££Ø×¢£ŗĀÆ
ŁĮĒ¦Ņ²”£”±øĆĮ¶Šæ¹¤ŅÕ¹ż³ĢÖ÷ŅŖ·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ____”££Ø×¢£ŗĀÆ øŹŹÆµÄÖ÷ŅŖ³É·ÖĪŖĢ¼ĖįŠæ£¬ŁĮĒ¦ŹĒÖø½šŹōŠæ£©
øŹŹÆµÄÖ÷ŅŖ³É·ÖĪŖĢ¼ĖįŠæ£¬ŁĮĒ¦ŹĒÖø½šŹōŠæ£©

| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ĶŗĶĆ¾µÄŗĻ½š4.6 gĶźČ«ČÜÓŚÅØĻõĖį£¬Čō·“Ó¦ŗóĻõĖį±»»¹ŌÖ»²śÉś4 480 mLµÄNO2ĘųĢåŗĶ336 mLµÄN2O4ĘųĢå(¶¼ŅŃÕŪĖćµ½±ź×¼×“æö)£¬ŌŚ·“Ó¦ŗóµÄČÜŅŗÖŠ£¬¼ÓČė×ćĮæµÄĒāŃõ»ÆÄĘČÜŅŗ£¬Éś³É³ĮµķµÄÖŹĮæĪŖ (””””)
A£®9.02 g B£®8.51 g
C£®8.26 g D£®7.04g
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
(1)»š·ØĮ¶ŠæŹĒ½«ÉĮŠææó(Ö÷ŅŖ³É·ÖŹĒZnS)Ķعżø”Ń”±ŗÉÕŹ¹Ėü×Ŗ»ÆĪŖŃõ»ÆŠæ£¬ŌŁ°ŃŃõ»ÆŠæŗĶ½¹Ģæ»ģŗĻ£¬ŌŚ¹Ä·ēĀÆÖŠ¼ÓČȵ½1 100”«1 300 ”ę£¬Ź¹ŠæÕōĮó³öĄ“”£
¢ŁŠ“³ö»š·ØĮ¶ŠæµÄÖ÷ŅŖ·“Ó¦£ŗ
±ŗÉÕ·“Ó¦£ŗ_________________________________________”£
¹Ä·ēĀÆÖŠæÉÄÜ·¢ÉśµÄ·“Ó¦£ŗ___________________(ČĪŠ“Ņ»øö)
¢Ś“Ó±£»¤»·¾³ŗĶ³ä·ÖĄūÓĆŌĮĻ½Ē¶Čæ“ČēŗĪ“¦ĄķŗĶĄūÓĆ²śÉśµÄŃĢĘų£æ_____________________________________________________________________________”£
(2)¹¤ŅµÉĻŅ±Į¶ĀĮ¾ĶŹĒµē½āŃõ»ÆĀĮ
¢ŁŅ±Į¶ĀĮµÄµē½ā²ŪÖŠµÄŅõ¼«ŗĶŃō¼«²ÄĮĻ¾łÓĆŹÆÓĶĮ¶ÖĘŗĶĆŗµÄøÉĮó²śĘ·________(ĢīĪļÖŹĆū³Ę)”£
¢ŚŃõ»ÆĀĮµÄČŪµćŗÜøߣ¬ŌŚĀĮµÄŅ±Į¶ÖŠŅŖ¼ÓČė±ł¾§ŹÆ(Na3AlF6)£¬Ęä×÷ÓĆŹĒ________________________________________________”£
¢Ū¹¤ŅµÉĻŅ±Į¶ĀĮŹ±ÓƵÄŌĮĻŹĒAl2O3£¬¶ų²»ŹĒAlCl3£¬ĘäŌŅņŹĒ
______________________________________________________ӣ
(3)¹¤ŅµÉĻ”°ĮŖŗĻÖĘ¼ī·Ø”±ÖŠÖ÷ŅŖ·“Ó¦µÄ»Æѧ·½³ĢŹ½ŹĒ_______________________________________”£
ĘäÖŠµÄCO2Ą“Ō“ÓŚ______________”£
(4)Ģ¼ĖįøĘŹĒÖĘ²£Į§µÄŌĮĻÖ®Ņ»£¬¹¤ŅµÉĻÖĘ²£Į§ŹĒŌŚ²£Į§ČŪĀÆÖŠ½ųŠŠ£¬ĘäÖŠ·“Ó¦Ö®Ņ»ĪŖCaCO3£«SiO2CaSiO3£«CO2”ü£¬ČōŌŚÉĻŹöĢõ¼žĻĀ£¬°Ń1 000a g CaCO3ŗĶ60a g SiO2»ģŗĻ£¬ŌņÉś³ÉµÄCO2ŌŚ±ź×¼×“æöĻĀµÄĢå»żĪŖ________________(ÓĆŗ¬aµÄ“śŹżŹ½±ķŹ¾)”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
µēŹÆ½¬ŹĒĀČ¼ī¹¤ŅµÖŠµÄŅ»ÖÖ·ĻĘśĪļ£¬Ęä“óÖĀ×é³ÉČēĻĀ±ķĖłŹ¾£ŗ
| ³É·Ö | CaO | SiO2 | Al2O3 | Fe2O3 | MgO | CaS | ĘäĖū²»ČÜÓŚĖįµÄĪļÖŹ |
| ÖŹĮæ·ÖŹż (%) | 65”«66 | 3.5”«5.0 | 1.5”«3.5 | 0.2”«0.8 | 0.2”«1.1 | 1.0”«1.8 | 23”«26 |
ÓƵēŹÆ½¬æÉÉś²śĪŽĖ®CaCl2£¬Ä³»Æ¹¤³§Éč¼ĘĮĖŅŌĻĀ¹¤ŅÕĮ÷³Ģ£ŗ
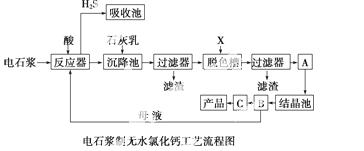
ŅŃÖŖĀČ»ÆøĘ¾§ĢåµÄ»ÆѧŹ½ŹĒCaCl2·6H2O£»H2SŹĒŅ»ÖÖĖįŠŌĘųĢ壬ĒŅ¾ßÓŠ»¹ŌŠŌ”£
(1)·“Ó¦Ę÷ÖŠ¼ÓČėµÄĖįӦєÓĆ ________”£
(2)ĶŃÉ«²ŪÖŠÓ¦¼ÓČėµÄĪļÖŹXŹĒ______________£»Éč±øAµÄ×÷ÓĆŹĒ______________£»Éč±øBµÄĆū³ĘĪŖ ______________£»Éč±øCµÄ×÷ÓĆŹĒ ____________”£
(3)ĪŖĮĖĀś×ć»·±£ŅŖĒó£¬Šč½«·ĻĘųH2SĶØČėĪüŹÕ³Ų£¬ĻĀĮŠĪļÖŹÖŠ×īŹŹŗĻ×÷ĪŖĪüŹÕ¼ĮµÄŹĒ________”£
A£®Ė® B£®ÅØĮņĖį C£®ŹÆ»ŅČé D£®ĻõĖį
(4)½«Éč±øBÖŠ²śÉśµÄÄøŅŗÖŲŠĀŅżČė·“Ó¦Ę÷µÄÄæµÄŹĒ__________________________
______________________ӣ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ÓÉČŪŃĪµē½ā·Ø»ńµĆµÄ“ÖĀĮŗ¬ÓŠŅ»¶ØĮæµÄ½šŹōÄĘŗĶĒāĘų£¬ÕāŠ©ŌÓÖŹæɲÉÓĆ“µĘų¾«Į¶·Ø³żČ„£¬²śÉśµÄĪ²Ęų¾“¦ĄķŗóæÉÓĆøֲĶĘĀĮ”£¹¤ŅÕĮ÷³ĢČēĻĀ£ŗ

(×¢£ŗNaClČŪµćĪŖ801 ”ę£»AlCl3ŌŚ181 ”ęÉż»Ŗ)
(1)¾«Į¶Ē°£¬ŠčĒå³żŪįŪö±ķĆęµÄŃõ»ÆĢśŗĶŹÆӢɰ£¬·ĄÖ¹¾«Į¶Ź±ĖüĆĒ·Ö±šÓėĀĮ·¢ÉśÖĆ»»·“Ó¦²śÉśŠĀµÄŌÓÖŹ£¬Ļą¹ŲµÄ»Æѧ·½³ĢŹ½ĪŖ¢Ł_____________ŗĶ
¢Ś___________________”£
(2)½«Cl2Į¬ŠųĶØČėŪįŪöÖŠµÄ“ÖĀĮČŪĢ壬ŌÓÖŹĖęĘųÅŻÉĻø”³żČ„”£ĘųÅŻµÄÖ÷ŅŖ³É·Ö³żCl2Ķā»¹ŗ¬ÓŠ_____________£»
¹ĢĢ¬ŌÓÖŹÕ³ø½ÓŚĘųÅŻÉĻ£¬ŌŚČŪĢå±ķĆęŠĪ³Éø”Ōü£¬ø”ŌüÖŠæĻ¶Ø“ęŌŚ________________”£
(3)ŌŚÓĆ·Ļ¼īŅŗ“¦ĄķAµÄ¹ż³ĢÖŠ£¬Ėł·¢Éś·“Ó¦µÄĄė×Ó·½³ĢŹ½ĪŖ_____________”£
(4)øֲĶĘĀĮŗ󣬱ķĆęŠĪ³ÉµÄÖĀĆÜŃõ»ÆĀĮĤÄÜ·ĄÖ¹øÖ²ÄøÆŹ“£¬ĘäŌŅņŹĒ____________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ĻĀĮŠŠšŹöÕżČ·µÄŹĒ
A£®ŗĻ³É°±µÄ”°ŌģĘų”±½×¶Ī»į²śÉś·ĻĘų
B£®µē¶ĘµÄĖįŠŌ·ĻŅŗÓĆ¼īÖŠŗĶŗó¾ĶæÉŅŌÅÅ·Å
C£®µē½āÖĘĀĮµÄ¹ż³ĢÖŠ£¬×÷ĪŖŃō¼«²ÄĮĻµÄĪŽŃĢĆŗ²»»įĻūŗÄ
D£®Ź¹ÓĆĆŗĢæ×Ŗ»ÆµÄ¹ÜµĄĆŗĘų±ČÖ±½ÓČ¼ĆŗæɼõÉŁ»·¾³ĪŪČ¾
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
¹¤ŅµÉĻÉś²śĮņĖįµÄĮ÷³ĢĶ¼ČēĻĀ£ŗ

Ēė»Ų“šĻĀĮŠĪŹĢā£ŗ
(1)ŌēĘŚÉś²śĮņĖįŅŌ»ĘĢśæóĪŖŌĮĻ£¬µ«ĻÖŌŚ¹¤³§Éś²śĮņĖįŅŌĮņ»ĘĪŖŌĮĻ£¬ĄķÓÉŹĒ________________________________________________________________________”£
(2)ŌŚĘųĢå½ųČė“߻Ʒ“Ó¦ŹŅĒ°Šč¾»»ÆµÄŌŅņŹĒ_________________________ ________
________
_____________________ ___________________________________________________ӣ
___________________________________________________ӣ
(3)ŌŚ“߻Ʒ“Ó¦ŹŅÖŠĶس£Ź¹ÓĆ³£Ń¹£¬ŌŚ“ĖĢõ¼žĻĀSO2µÄ×Ŗ»ÆĀŹĪŖ90%”£µ«ŹĒ²æ·Ö·¢“ļ¹ś¼Ņ²ÉČ”øßŃ¹Ģõ¼žĻĀÖĘČ”SO3£¬²ÉČ”¼ÓŃ¹“ėŹ©µÄÄæµÄ³żĮĖ¼Óæģ·“Ó¦ĖŁĀŹĶā£¬»¹æÉŅŌ____________________________£¬“Ó¶ųĢįøßÉś²śŠ§ĀŹ”£
(4)¹¤ŅµÉś²śÖŠ³£ÓĆ°±—Ėį·Ø½ųŠŠĪ²ĘųĶŃĮņ£¬ŅŌ“ļµ½Ļū³żĪŪČ¾£¬·ĻĪļĄūÓƵÄÄæµÄ”£ÓĆ»Æѧ·½³ĢŹ½±ķŹ¾Ęä·“Ó¦ŌĄķ£ŗ____________________________________________________
________________________________________________________________________
________________________________________________________________________ӣ
(5)³żĮņĖį¹¤ŅµĶā£¬»¹ÓŠŠķ¶ą¹¤ŅµÉś²ś”£ĻĀĮŠĻą¹ŲµÄ¹¤ŅµÉś²śĮ÷³ĢÖŠÕżČ·µÄŹĒ________”£
A£®ŗ£Ė®Ģįäå£ŗŗ£Ė®ÅØĖõ
 äåÕōĘų
äåÕōĘų
 Ņŗäå
Ņŗäå
B£®ŗ£Ė®ĢįĆ¾£ŗŗ£Ģ²±“æĒ
 ŹÆ»ŅĖ®
ŹÆ»ŅĖ®
 MgO
MgO Ć¾
Ć¾
C£®¹¤ŅµÖĘĻõĖį£ŗæÕĘų NO2
NO2 ĻõĖį”Ŗ”śĪ²Ęų“¦Ąķ
ĻõĖį”Ŗ”śĪ²Ęų“¦Ąķ
D£®¹¤ŅµŗĻ³É°±£ŗĢģČ»Ęų ĒāĘų
ĒāĘų NH3”¢H2”¢N2
NH3”¢H2”¢N2 °±
°±
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ŌŖĖŲH”¢C”¢N”¢O”¢F¶¼ŹĒÖŲŅŖµÄ·Ē½šŹōŌŖĖŲ,Fe”¢CuŹĒÓ¦ÓĆ·Ē³£¹ć·ŗµÄ½šŹō”£
(1)FeŌŖĖŲ»łĢ¬Ō×ÓµÄŗĖĶāµē×ÓÅŲ¼Ź½ĪŖ”””””””””£
(2)C”¢HŌŖĖŲŠĪ³ÉµÄ»ÆŗĻĪļ·Ö×ÓÖŠ¹²ÓŠ16øöµē×Ó,øĆ·Ö×ÓÖŠ¦Ņ¼üÓė¦Š¼üµÄøöŹż±ČĪŖ”””””””””£
(3)C”¢N”¢OČżÖÖŌŖĖŲµÄµŚŅ»µēĄėÄÜÓɓ󵽊”µÄĖ³ŠņĪŖ(ÓĆŌŖĖŲ·ūŗűķŹ¾)”””””””””£
(4)ŌŚ²ā¶ØHFµÄĻą¶Ō·Ö×ÓÖŹĮæŹ±,ŹµŃé²āµĆÖµŅ»°ćøßÓŚĄķĀŪÖµ,ĘäÖ÷ŅŖŌŅņŹĒ
”””£
(5)C”¢NĮ½ŌŖĖŲŠĪ³ÉµÄ»ÆŗĻĪļC3N4ŠĪ³ÉµÄŌ×Ó¾§Ģå,½į¹¹ĄąĖĘ½šøÕŹÆ,ÉõÖĮÓ²¶Č³¬¹ż½šøÕŹÆ,ĘäŌŅņŹĒ
”””£
(6)ČēĶ¼ĪŖŹÆÄ«¾§°ū½į¹¹Ź¾ŅāĶ¼,øĆ¾§°ūÖŠŗ¬ÓŠCŌ×ÓµÄøöŹżĪŖ”””””””””£

²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ĻĀĮŠĖµ·ØÕżČ·µÄŹĒ(NAĪŖ°¢·ü¼ÓµĀĀŽ³£ŹżµÄÖµ)(””””)
A£®³£ĪĀ³£Ń¹ĻĀ£¬22.4 L CO2ÖŠŗ¬ÓŠNAøöCO2·Ö×Ó
B£®±ź×¼×“æöĻĀ£¬22.4 LæÕĘųŗ¬ÓŠNAøöµ„ÖŹ·Ö×Ó
C£®22.4 L Cl2ÖŠŗ¬ÓŠNAøöCl2·Ö×Ó
D£®±ź×¼×“æöĻĀ£¬1.12 L O2ŗĶ1.12 L CO 2¾łŗ¬0.1NAøöŃõŌ×Ó
2¾łŗ¬0.1NAøöŃõŌ×Ó
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com