
��֪FeSO4��7H2O�����ڼ��������·������·�Ӧ��
2FeSO4��7H2O Fe2O3
+ SO2��+SO3��+14H2O������ͼ��װ�ÿ���������������Ӧ�����е���������ش��������⣺
Fe2O3
+ SO2��+SO3��+14H2O������ͼ��װ�ÿ���������������Ӧ�����е���������ش��������⣺
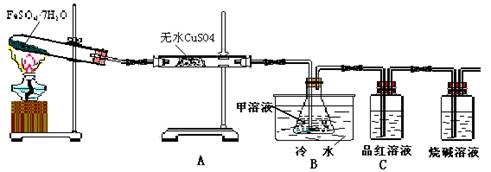
��1�����ڼ���SO2�����װ���� ����װ�õ���ĸ����ȷ��ˮ�������ڵ������� ��
��2������װ��B����ȷ�ϵIJ����� ��װ��B�еļ���Һ��ѡ(�����) ��
����������ʯ��ˮ �������Ȼ�����Һ ���������ᱵ��Һ
��װ������ˮ�������� ��
��3��Ϊ̽��Fe2O3�����ʺ���;��ȡ�Թ��������������������������Ʊ�����������Һ�����˱�����Һ��μ����ˮ�м���Ƭ�̣��۲�Һ�����ɫ��Ϊ ������÷�ɢϵ�����Ϊ ��
 ���ɶ���ܲ��¿�ֱͨ�п�ϵ�д�
���ɶ���ܲ��¿�ֱͨ�п�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ||

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| O | - 4 |
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011������ʡ������һ�и�����ѧ�������Ի�ѧ�Ծ� ���ͣ�ʵ����
��֪FeSO4��7H2O�����ڼ��������·������·�Ӧ��
2FeSO4��7H2O Fe2O3+SO2��+SO3��+14H2O������ͼ��װ�ÿ���������������Ӧ�����е���������ش��������⣺
Fe2O3+SO2��+SO3��+14H2O������ͼ��װ�ÿ���������������Ӧ�����е���������ش��������⣺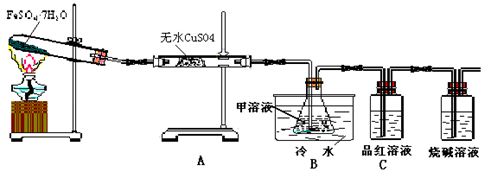
��1�����ڼ���SO2�����װ���� ����װ�õ���ĸ����ȷ��ˮ�������ڵ������� ��
��2������װ��B����ȷ�ϵIJ����� ��װ��B�еļ���Һ��ѡ(�����) ��
����������ʯ��ˮ �������Ȼ�����Һ ���������ᱵ��Һ
��װ������ˮ�������� ��
��3��Ϊ̽��Fe2O3�����ʺ���;��ȡ�Թ��������������������������Ʊ�����������Һ�����˱�����Һ��μ����ˮ�м���Ƭ�̣��۲�Һ�����ɫ��Ϊ ������÷�ɢϵ�����Ϊ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ�����ʡ�����и����ڶ�������������ۻ�ѧ�Ծ��������棩 ���ͣ�ʵ����
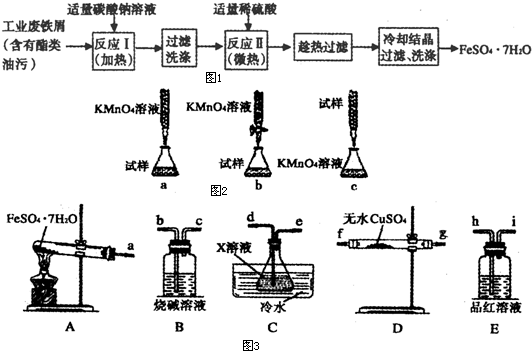
FeSO4��7H2O�㷺����ҽҩ��ҵ����
��1���������Թ�ҵ����мΪԭ������FeSO4��7H2O������ͼ��

����д���пհס�
�ټ�����̼������Һ��Ŀ����______________����ӦI��Ҫ���������ӣ���ԭ����___________��
���жϷ�Ӧ����ɵ�������__________����Ӧ����Ҫ100mL1mol��L��ϡ���ᣬ��98��3�����ѣ�1��84g��cm3��Ũ�������ơ����õ���������Ͳ���ձ�������������ͷ�ιܼ�____________.
�۲ⶨFeSO4��7H2O��Ʒ��Fe2�������ķ�������KMnO4��Һ�ζ�����5Fe2���� ��8H����5Fe3����Mn2����4H2O��������Ϊ��
��8H����5Fe3����Mn2����4H2O����������
��ȡ2��8500g FeSO4��7H2O��Ʒ�����Ƴ�250mL��Һ��
����ȡ25��00mL������Һ����ƿ�У�
���������ữ��0��01000moL��L KMnO4��Һ�ζ����յ㣬����KMnO4��Һ�����ƽ��ֵΪ20��00mL��

ijͬѧ�����ͼ��ʾ�ĵζ���ʽ�У����������____________���гֲ�����ȥ��������ĸ��ţ����жϴ˵ζ�ʵ��ﵽ�յ�ķ�����____________������������Ʒ��FeSO4��7H2O����������Ϊ________����С����ʾ��������λС�������������������ⶨ����Ʒ��FeSO4��7H2O����������ƫ�ͣ��ⶨ�����в��������ɺ��ԣ��������ԭ����_____________��______________��
��2����֪FeSO4��7H2O�����ڼ��������·������·�Ӧ��2FeSO4��7H2O Fe2O3��SO2����SO3����14H2O����������ͼװ�ÿɼ���÷�Ӧ��������
Fe2O3��SO2����SO3����14H2O����������ͼװ�ÿɼ���÷�Ӧ��������

����д���пհס�
������������˳��Ϊa��________��__________��_________��________��__________��__________��_____________��
��װ��C�е�XΪ______________����װ������ˮ��������_________________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com