
���� ��ȡ����Һ�������Թ��У��μ�����ϡHNO3���������ݣ�˵������CO32-����һ����������CO32-��Ӧ��Ca2+��Cu2+������Һ�ĵ�����ԭ���֪����Na+��
����ٵ���Һ�м���AgNO3��Һ���а�ɫ�������ɣ�˵������Cl-���Դ˽����⣮
��� �⣺��ȡ����Һ�������Թ��У��μ�����ϡHNO3���������ݣ�˵������CO32-����һ����������CO32-��Ӧ��Ca2+��Cu2+������Һ�ĵ�����ԭ���֪����Na+��
����ٵ���Һ�м���AgNO3��Һ���а�ɫ�������ɣ�˵������Cl-��NO3-����ȷ���Ƿ���ڣ�
�ʴ�Ϊ��Na+��CO32-��Cl-��Ca2+��Cu2+��NO3-��
���� ���⿼�����ӵļ���Ϊ��Ƶ���㣬���ط�����Ӧ�������Ŀ��飬ע��������ʵ������Լ���Һ�����Ե�Ӧ�ã���Ŀ�ѶȲ���



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| ѡ�� | Ӧ�� | ���� |
| A | �ý��ݹ����������Һ�Ĺ���������ˮ�� | ������������ˮ���ͷų�����ϩ |
| B | ���뺣�ڵĸ���բ����װһ��������п���ֹբ�ű���ʴ | ������ӵ����������������������� |
| C | ��������������Ͻ���� | ������������Ӧ |
| D | �ߴ����������оƬ�IJ��� | �辧������Ȼ�������ȶ����� |
| A�� | A | B�� | B | C�� | C | D�� | D |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �轺������ʳƷ�Ŀ������� | |
| B�� | �����ȼ�ˮ��ϴ�����ϵ����� | |
| C�� | �������п����ǿ�俹��ʴ�� | |
| D�� | ����ȼ�ջ�ʯȼ���ǵ���������������Ҫ����֮һ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
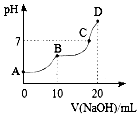 25��ʱ����10mL 0.1mol•L-1 H2A��Һ�еμӵ�Ũ�ȵ�NaOH��Һ����Һ��pH��NaOH��Һ�������ϵ��ͼ��ʾ����������������ǣ�������
25��ʱ����10mL 0.1mol•L-1 H2A��Һ�еμӵ�Ũ�ȵ�NaOH��Һ����Һ��pH��NaOH��Һ�������ϵ��ͼ��ʾ����������������ǣ�������| A�� | C����Һ�к���NaHA��Na2A | |
| B�� | NaHA��Һ��ˮ�ĵ���̶ȱ�Na2A��Һ��С | |
| C�� | B�㣬c ��Na+��=2[c ��H2A��+c ��HA��+c ��A2-��] | |
| D�� | D�㣬c ��Na+����c ��A2-����c ��OH-����c ��HA-����c ��H+�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
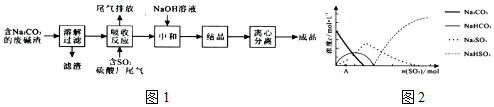
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �٢ڢۢݢ� | B�� | �ڢۢܢ� | C�� | �٢ڢۢܢ� | D�� | �٢ڢۢܢ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �����е���������ԭ�Ӷ������� | |
| B�� | CH2Cl2�Ǵ�����˵��������������ṹ�����������νṹ | |
| C�� | ��Ȼ�����������ӵ�������Ҫ�ɷ־��Ǽ��� | |
| D�� | ����ͼ���һ��������������ȡ����Ӧ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ʵ�����ϵľƾ����㵹��ȼ��������Ӧ������ˮ���� | |
| B�� | ��Һ����ʱ����Һ©���е��²�Һ����¿ڷų����ٽ��ϲ�Һ����¿ڷų�����һ�ձ��� | |
| C�� | ���Թܼд��Թܵ��������ϼ�ס����ܿ�Լ$\frac{1}{3}$�����ֳ��Թܼг���ĩ�˽��м��� | |
| D�� | Ϊ����ij��Һ���Ƿ���NH4+�����ڴ���Һ�м���������NaOH��Һ�����Ⱥ���ʪ�����ɫʯ����ֽ���� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com