
��14�֣��ϳ�������H2��CO��Ϊ��Ҫ��ɵĹ���ѧ�ϳɵ�ԭ�������ش��й����⣺
��1��������һ����������ˮ��Ӧ�ɵúϳ������÷�Ӧ��ÿת��1��2mol���ӣ����úϳ����ڱ����µ����Ϊ L��
��2���ϳ����ںϳɰ���ʱ���ȥCO�����������·�Ӧ�� CO��g��+H2O��g�� CO2��g����H2��g��
CO2��g����H2��g��  H=-41��16kJ��mol�����ڸ÷�Ӧ�йر�����ȷ���� ��
H=-41��16kJ��mol�����ڸ÷�Ӧ�йر�����ȷ���� ��
a�������������䣬��С��ϵ�������ѹǿʱ��Ӧ���ʲ��䣬����ƽ�ⲻ�ƶ�
b�����������£�����ø���ϵ�¶Ȳ��ٸı䣬��Ӧ����ƽ��״̬
c���¶����ߣ��÷�Ӧƽ�ⳣ������
d��Ϊ���COת���ʣ����ʵ�����ˮ��������
ij�¶��£�H2O��CO�������5��l����ij���ݷ�Ӧ����ƽ��ʱ���CO��CO2�����l��9����������µ�ƽ�ⳣ��Ϊ ��������λ��Ч���֣�
��3��Ϊ��ȥ��2�������ɵ�CO2������̼�����Һ���գ�������pH=10��̼�����Һ��ˮ�����OH- �����ʵ���Ũ��Ϊ ��̼�������Һ������Ũ�ȴ�С˳��Ϊ
��4��CO2��NH3��һ�������·�Ӧ�������أ�CO��NH2��2�ݣ�CO2��g��+2NH3��1����H2O��1��+CO��NH2��2��1����CO2��ת�������¶ȱ仯�������£���÷�Ӧ H 0����������������Ӻϳ����ڳ������������Ժ���һ������CO2��NH3��Ӧ��δ��� ��
H 0����������������Ӻϳ����ڳ������������Ժ���һ������CO2��NH3��Ӧ��δ��� ��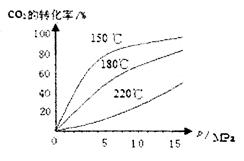

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��14�֣��ϳ�������H2��CO��Ϊ��Ҫ��ɵĹ���ѧ�ϳɵ�ԭ�������ش��й����⣺
��1��������һ����������ˮ��Ӧ�ɵúϳ������÷�Ӧ��ÿת��1��2mol���ӣ����úϳ����ڱ����µ����Ϊ L��
��2���ϳ����ںϳɰ���ʱ���ȥCO�����������·�Ӧ�� CO��g��+H2O��g��![]() CO2��g����H2��g��
CO2��g����H2��g�� ![]() H=-41��16kJ��mol�����ڸ÷�Ӧ�йر�����ȷ���� ��
H=-41��16kJ��mol�����ڸ÷�Ӧ�йر�����ȷ���� ��
a�������������䣬��С��ϵ�������ѹǿʱ��Ӧ���ʲ��䣬����ƽ�ⲻ�ƶ�
b�����������£�����ø���ϵ�¶Ȳ��ٸı䣬��Ӧ����ƽ��״̬
c���¶����ߣ��÷�Ӧƽ�ⳣ������
d��Ϊ���COת���ʣ����ʵ�����ˮ��������
ij�¶��£�H2O��CO�������5��l����ij���ݷ�Ӧ����ƽ��ʱ���CO��CO2�����l��9����������µ�ƽ�ⳣ��Ϊ ��������λ��Ч���֣�
��3��Ϊ��ȥ��2�������ɵ�CO2������̼�����Һ���գ�������pH=10��̼�����Һ��ˮ�����OH- �����ʵ���Ũ��Ϊ ��̼�������Һ������Ũ�ȴ�С˳��Ϊ
��4��CO2��NH3��һ�������·�Ӧ�������أ�CO��NH2��2�ݣ�CO2��g��+2NH3��1����H2O��1��+CO��NH2��2��1����CO2��ת�������¶ȱ仯�������£���÷�Ӧ![]() H 0����������������Ӻϳ����ڳ������������Ժ���һ������CO2��NH3��Ӧ��δ��� ��
H 0����������������Ӻϳ����ڳ������������Ժ���һ������CO2��NH3��Ӧ��δ��� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��14�֣��ϳ�������H2��CO��Ϊ��Ҫ��ɵĹ���ѧ�ϳɵ�ԭ�������ش��й����⣺
��1��������һ����������ˮ��Ӧ�ɵúϳ������÷�Ӧ��ÿת��1��2mol���ӣ����úϳ����ڱ����µ����Ϊ L��
��2���ϳ����ںϳɰ���ʱ���ȥCO�����������·�Ӧ�� CO��g��+H2O��g��![]() CO2��g����H2��g��
CO2��g����H2��g�� ![]() H=-41��16kJ��mol�����ڸ÷�Ӧ�йر�����ȷ���� ��
H=-41��16kJ��mol�����ڸ÷�Ӧ�йر�����ȷ���� ��
a�������������䣬��С��ϵ�������ѹǿʱ��Ӧ���ʲ��䣬����ƽ�ⲻ�ƶ�
b�����������£�����ø���ϵ�¶Ȳ��ٸı䣬��Ӧ����ƽ��״̬
c���¶����ߣ��÷�Ӧƽ�ⳣ������
d��Ϊ���COת���ʣ����ʵ�����ˮ��������
ij�¶��£�H2O��CO�������5��l����ij���ݷ�Ӧ����ƽ��ʱ���CO��CO2�����l��9����������µ�ƽ�ⳣ��Ϊ ��������λ��Ч���֣�
��3��Ϊ��ȥ��2�������ɵ�CO2������̼�����Һ���գ�������pH=10��̼�����Һ��ˮ�����OH- �����ʵ���Ũ��Ϊ ��̼�������Һ������Ũ�ȴ�С˳��Ϊ
��4��CO2��NH3��һ�������·�Ӧ�������أ�CO��NH2��2�ݣ�CO2��g��+2NH3��1����H2O��1��+CO��NH2��2��1����CO2��ת�������¶ȱ仯�������£���÷�Ӧ![]() H 0����������������Ӻϳ����ڳ������������Ժ���һ������CO2��NH3��Ӧ��δ��� ��
H 0����������������Ӻϳ����ڳ������������Ժ���һ������CO2��NH3��Ӧ��δ��� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ��ɽ��ʡ����3���¿����۲��֣������棩 ���ͣ������
��14�֣��ϳ�������H2��CO��Ϊ��Ҫ��ɵĹ���ѧ�ϳɵ�ԭ�������ش��й����⣺
��1��������һ����������ˮ��Ӧ�ɵúϳ������÷�Ӧ��ÿת��1��2mol���ӣ����úϳ����ڱ����µ����Ϊ L��
��2���ϳ����ںϳɰ���ʱ���ȥCO�����������·�Ӧ�� CO��g��+H2O��g�� CO2��g����H2��g��
CO2��g����H2��g��  H=-41��16kJ��mol�����ڸ÷�Ӧ�йر�����ȷ����
��
H=-41��16kJ��mol�����ڸ÷�Ӧ�йر�����ȷ����
��
a�������������䣬��С��ϵ�������ѹǿʱ��Ӧ���ʲ��䣬����ƽ�ⲻ�ƶ�
b�����������£�����ø���ϵ�¶Ȳ��ٸı䣬��Ӧ����ƽ��״̬
c���¶����ߣ��÷�Ӧƽ�ⳣ������
d��Ϊ���COת���ʣ����ʵ�����ˮ��������
ij�¶��£�H2O��CO�������5��l����ij���ݷ�Ӧ����ƽ��ʱ���CO��CO2�����l��9����������µ�ƽ�ⳣ��Ϊ ��������λ��Ч���֣�
��3��Ϊ��ȥ��2�������ɵ�CO2������̼�����Һ���գ�������pH=10��̼�����Һ��ˮ�����OH- �����ʵ���Ũ��Ϊ ��̼�������Һ������Ũ�ȴ�С˳��Ϊ
��4��CO2��NH3��һ�������·�Ӧ�������أ�CO��NH2��2�ݣ�CO2��g��+2NH3��1����H2O��1��+CO��NH2��2��1����CO2��ת�������¶ȱ仯�������£���÷�Ӧ H 0����������������Ӻϳ����ڳ������������Ժ���һ������CO2��NH3��Ӧ��δ���
��
H 0����������������Ӻϳ����ڳ������������Ժ���һ������CO2��NH3��Ӧ��δ���
��

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ��ɽ��ʡ�������Ĵ���Ͽ��������ۺ��Ծ�����ѧ���֣� ���ͣ������
��14�֣��ϳ�������H2��CO��Ϊ��Ҫ��ɵĹ���ѧ�ϳɵ�ԭ�������ش��й����⣺
��1��������һ����������ˮ��Ӧ�ɵúϳ������÷�Ӧ��ÿת��1��2mol���ӣ����úϳ����ڱ����µ����Ϊ L��
��2���ϳ����ںϳɰ���ʱ���ȥCO�����������·�Ӧ�� CO��g��+H2O��g�� CO2��g����H2��g��
CO2��g����H2��g�� 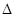 H=-41��16kJ��mol�����ڸ÷�Ӧ�йر�����ȷ����
��
H=-41��16kJ��mol�����ڸ÷�Ӧ�йر�����ȷ����
��
a�������������䣬��С��ϵ�������ѹǿʱ��Ӧ���ʲ��䣬����ƽ�ⲻ�ƶ�
b�����������£�����ø���ϵ�¶Ȳ��ٸı䣬��Ӧ����ƽ��״̬
c���¶����ߣ��÷�Ӧƽ�ⳣ������
d��Ϊ���COת���ʣ����ʵ�����ˮ��������
ij�¶��£�H2O��CO�������5��l����ij���ݷ�Ӧ����ƽ��ʱ���CO��CO2�����l��9����������µ�ƽ�ⳣ��Ϊ ��������λ��Ч���֣�
��3��Ϊ��ȥ��2�������ɵ�CO2������̼�����Һ���գ�������pH=10��̼�����Һ��ˮ�����OH- �����ʵ���Ũ��Ϊ ��̼�������Һ������Ũ�ȴ�С˳��Ϊ
��4��CO2��NH3��һ�������·�Ӧ�������أ�CO��NH2��2�ݣ�CO2��g��+2NH3��1����H2O��1��+CO��NH2��2��1����CO2��ת�������¶ȱ仯�������£���÷�Ӧ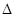 H 0����������������Ӻϳ����ڳ������������Ժ���һ������CO2��NH3��Ӧ��δ���
��
H 0����������������Ӻϳ����ڳ������������Ժ���һ������CO2��NH3��Ӧ��δ���
��

�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com