

 Na2 CO3 + H2O + CO2�� Na2CO3��10H2O
Na2 CO3 + H2O + CO2�� Na2CO3��10H2O  Na2CO3 + 10H2O
Na2CO3 + 10H2O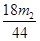 )/180m ��100%
)/180m ��100% Na2 CO3 + H2O + CO2����Na2CO3��10H2O
Na2 CO3 + H2O + CO2����Na2CO3��10H2O  Na2CO3 + 10H2O��
Na2CO3 + 10H2O��


| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A���������ۻ�ΪС�� | B������Ϊ��ɫ |
| C��ȼ�պ�õ�һ��ɫ���� | D��ȼ�պ�õ�����ɫ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
 X + CO2 + H2O�� �� Z + CO2
X + CO2 + H2O�� �� Z + CO2 X + O2 ��
X + O2 �� Y + O2 �� �� X + Ca(OH)2
Y + O2 �� �� X + Ca(OH)2 Y + CaCO3
Y + CaCO3 �鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��̼���� | B���������� | C�������� | D��̼�ᱵ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| ѡ�� | ������ | ������ | �ж� |
| A | ̼���ơ�̼���������ó���ʯ��ˮ���� | Na2CO2�������ʯ��ˮ��Ӧ���ɰ�ɫ��������NaHCO3���� | ��ԣ� ��ԣ��� |
| B | ��Na2O2��ˮ��Һ�е����̪���ɫ | Na2O2��ˮ��Ӧ������������ | ��ԣ� ������� |
| C | �����ƾ���ǿ��ԭ�� | ��ѹ�ƵƷ�������ǿ�Ļƹ� | ��ԣ� ��ԣ��� |
| D | ��̪��Һ��̼������Һ�е���ɫ����̼��������Һ���� | CO32��ˮ��̶ȴ���HCO3�� | ��ԣ� ��ԣ��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��5.28g | B��4.22g | C��3.18g | D��2.12g |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��������ˮ�е��ܽ�Ȳ�ͬ��̼�����Ƶ��ܽ�ȴ���̼���Ƶ��ܽ�� |
| B���������ȶ��Բ�ͬ��̼���Ƶ����ȶ��Դ���̼�����Ƶ����ȶ��� |
| C�����߶��������ᷴӦ�ų�������̼���壬���������ͷ�Ӧ������ͬ |
| D��������һ���������²����ת�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��Na2CO3 | B��Na2CO3��NaOH | C��NaHCO3 | D��NaHCO3��Na2CO3 |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com