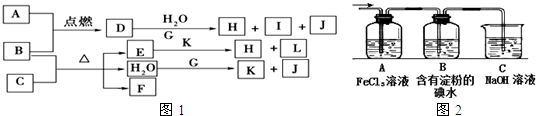
�⣺��J��Ԫ��ԭ�Ӻ���ֻ��һ������֪JΪH
2��I����ɫ��ӦΪ��ɫ������I��NaԪ�أ��ɽ���B��A������ȼ�ղ����ػ�ɫ�̿�֪AΪCl
2��BΪFe��Cu����DΪFeCl
3��CuCl
2����
��D+G+H
2O��H+I+J�����I��NaԪ��֪GΪ�����ƣ���H
2O+G��K+J��H
2��������KΪNaOH��D��ˮ��Һ���ػ�ɫ����DΪFeCl
3��
��ˮ��Һ��Na�ķ�ӦΪ��6Na+2FeCl
3+6H
2O�T2Fe��OH��
3��+6NaCl+3H
2����FΪ��ɫ���д̼�����ζ���壬����ʹƷ����Һ��ɫ��ӦΪSO
2��
��CΪH
2SO
4��EΪFe
2��SO
4��
3��HΪFe��OH��
3��LΪNaCl��
��1�������Ϸ�����֪��KΪNaOH��HΪFe��OH��
3���ʴ�Ϊ��NaOH��Fe��OH��
3��
��2�����Ȼ�����Һ�м�������ƣ����Ⱥ�ˮ��Ӧ�����������ƺ��������������ƺ��Ȼ�����Ӧ������������������
��Ӧ�Ļ�ѧ����ʽΪ2Fe
3++6H
2O+6Na=2Fe��OH��
3��+6Na
++3H
2����2Na+2H
2O�T2Na
++2OH
-+H
2����Fe
3++3OH
-=Fe��OH��
3����
�ʴ�Ϊ��2Fe
3++6H
2O+6Na=2Fe��OH��
3��+6Na
++3H
2����2Na+2H
2O�T2Na
++2OH
-+H
2����Fe
3++3OH
-=Fe��OH��
3����
��3��Fe
3+���Ӿ���ǿ�����ԣ�SO
2���л�ԭ�ԣ�����Һ�з�Ӧ����������ԭ��Ӧ�����������Ӻ���������ӣ���Ӧ�����ӷ���ʽΪ2Fe
3++SO
2+2H
2O=2Fe
2++SO
42-+4H
+��
�ʴ�Ϊ��2Fe
3++SO
2+2H
2O=2Fe
2++SO
42-+4H
+��
��4������������Ũ�����ڼ��������·�Ӧ�����������Ͷ��������ˮ����Ӧ�Ļ�ѧ����ʽΪ2Fe+6H
2SO
4��Ũ��

Fe
2��SO
4��
3+3SO
2��+6H
2O��
�ʴ�Ϊ��2Fe+6H
2SO
4��Ũ��

Fe
2��SO
4��
3+3SO
2��+6H
2O��
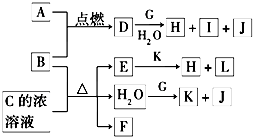
��������J��Ԫ��ԭ�Ӻ���ֻ��һ������֪JΪH
2��I����ɫ��ӦΪ��ɫ������I��NaԪ�أ��ɽ���B��A������ȼ�ղ����ػ�ɫ�̿�֪AΪCl
2��BΪFe��Cu����DΪFeCl
3��CuCl
2����
��D+G+H
2O��H+I+J�����I��NaԪ��֪GΪ�����ƣ���H
2O+G��K+J��H
2��������KΪNaOH��D��ˮ��Һ���ػ�ɫ����DΪFeCl
3����ˮ��Һ��Na�ķ�ӦΪ��
6Na+2FeCl
3+6H
2O�T2Fe��OH��
3��+6NaCl+3H
2����FΪ��ɫ���д̼�����ζ���壬����ʹƷ����Һ��ɫ��ӦΪSO
2����CΪH
2SO
4��EΪFe
2��SO
4��
3��HΪFe��OH��
3��LΪNaCl���Դ˽����⣮
���������⿼��������ƶϣ���Ŀ�Ѷ��еȣ�������Ĺؼ�����ȷ�ƶ����ʵ����࣬�������ʵ����ʽ��

 ��ͼ�У�A��LΪ�������ʻ�����ʵ�ˮ��Һ��B��A������ȼ�ղ����ػ�ɫ�̣�B��GΪ��ѧ��ѧ�г����������ʣ�I����ɫ��ӦΪ��ɫ��D����Һ���ػ�ɫ�����J��Ԫ��ԭ�Ӻ���ֻ��һ�����ӣ�FΪ��ɫ���д̼�����ζ���壬����ʹƷ����Һ��ɫ��
��ͼ�У�A��LΪ�������ʻ�����ʵ�ˮ��Һ��B��A������ȼ�ղ����ػ�ɫ�̣�B��GΪ��ѧ��ѧ�г����������ʣ�I����ɫ��ӦΪ��ɫ��D����Һ���ػ�ɫ�����J��Ԫ��ԭ�Ӻ���ֻ��һ�����ӣ�FΪ��ɫ���д̼�����ζ���壬����ʹƷ����Һ��ɫ�� Fe2��SO4��3+3SO2��+6H2O��
Fe2��SO4��3+3SO2��+6H2O�� Fe2��SO4��3+3SO2��+6H2O��
Fe2��SO4��3+3SO2��+6H2O��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
 ��ͼ�У�A��LΪ�������ʻ�����ʵ�ˮ��Һ��B��A������ȼ�ղ����ػ�ɫ�̣�B��GΪ��ѧ��ѧ�г����������ʣ�I����ɫ��ӦΪ��ɫ��D����Һ���ػ�ɫ�����J��Ԫ��ԭ�Ӻ���ֻ��һ�����ӣ�FΪ��ɫ���д̼�����ζ���壬����ʹƷ����Һ��ɫ��
��ͼ�У�A��LΪ�������ʻ�����ʵ�ˮ��Һ��B��A������ȼ�ղ����ػ�ɫ�̣�B��GΪ��ѧ��ѧ�г����������ʣ�I����ɫ��ӦΪ��ɫ��D����Һ���ػ�ɫ�����J��Ԫ��ԭ�Ӻ���ֻ��һ�����ӣ�FΪ��ɫ���д̼�����ζ���壬����ʹƷ����Һ��ɫ�� �ش��������⣺
�ش��������⣺ ��ͼ�У�A��LΪ�������ʻ�����ʵ�ˮ��Һ��B��A������ȼ�ղ����ػ�ɫ�̣�B��GΪ��ѧ��ѧ�г����������ʣ�I����ɫ��ӦΪ��ɫ��D����Һ���ػ�ɫ�����J��Ԫ��ԭ�Ӻ���ֻ��һ�����ӣ�FΪ��ɫ���д̼�����ζ���壬����ʹƷ����Һ��ɫ��
��ͼ�У�A��LΪ�������ʻ�����ʵ�ˮ��Һ��B��A������ȼ�ղ����ػ�ɫ�̣�B��GΪ��ѧ��ѧ�г����������ʣ�I����ɫ��ӦΪ��ɫ��D����Һ���ػ�ɫ�����J��Ԫ��ԭ�Ӻ���ֻ��һ�����ӣ�FΪ��ɫ���д̼�����ζ���壬����ʹƷ����Һ��ɫ��