
| ���� | CO | H2 | CH3OH |
| Ũ�ȣ�mol?L-1�� | 0.9 | 1.0 | 0.6 |
| ������ |
| �����ʵ��� |
| 1.8mol��28g/mol+2mol��28g/mol+1.2mol��2g/mol |
| 1.8mol+2mol+1.2mol |
| 0.6 |
| 0.9��1��02 |
| 0.6 |
| 0.9��1��02 |
| 1 |
| 1.5��12 |


 ��У��ʦ������ҵ���Ӻ����Ծ�ϵ�д�
��У��ʦ������ҵ���Ӻ����Ծ�ϵ�д� ȫ�̽��ϵ�д�
ȫ�̽��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
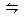 p C(g)���ﵽƽ��������������ѹ����ԭ����һ�룬���ٴ�ƽ��ʱC����Һ��ԭ����1.9����������������ȷ���� ( )
p C(g)���ﵽƽ��������������ѹ����ԭ����һ�룬���ٴ�ƽ��ʱC����Һ��ԭ����1.9����������������ȷ���� ( )| A��ƽ��������Ӧ�����ƶ� | B��C����������������� |
| C������A��ת���ʽ��� | D��m��n��p |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ��ʴ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ��ʴ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ��ʴ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ��ʴ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ��ʴ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��v��C��=v��D��=0.25mol?L-1?s-1 |
| B��z=2 |
| C��B��ת����Ϊ25% |
| D��C���������Ϊ28.6% |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��ֻ�Т� | B��ֻ�Тۢܢ� | C��ֻ�Т٢ڢۢ� | D�������� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com