
�췯�ƣ��ظ����ƣ�Na2Cr2O7?2H2O������Ҫ�Ļ�������ԭ�ϣ���ӡȾ��ҵ����ƹ�ҵ��Ƥ�﹤ҵ�����������ڻ�ѧ��ҵ����ҩ��ҵ��Ҳ������������Ӧ������ʮ�ֹ㷺��
��1��ʵ�����к췯�ƿ��ø�������Ҫ�ɷ֣�FeO?Cr2O3���������¹�������ȡ��
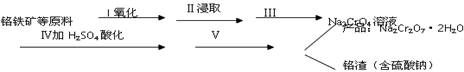
�١�����I�з�Ӧ�Ļ�ѧ����ʽΪ��
![]() ���÷�Ӧ�Ļ�ѧƽ�ⳣ���ı���ʽΪ ��
���÷�Ӧ�Ļ�ѧƽ�ⳣ���ı���ʽΪ ��
�ڲ�����з�����Ӧ�����ӷ���ʽΪ�� ��
��2�����췯����KCl����1��2�����ʵ����ȣ��������ˮ���ʵ������ɵõ�K2Cr2O7���塣����д���пո�����������̡�
| ��� | ʵ�鲽�� | ����ʵ��������������������װʵ��װ�ã� |
| �� | �ܽ� | �����������ձ��У���ˮ����ֽ���ֱ�����岻���ܽ⡣ |
| �� |
| |
| �� |
| |
| �� |
| |
| �� | ���ˡ����� | �õ�K2Cr2O7���� |

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
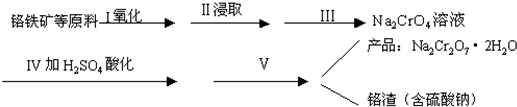
| c(CO2)8 |
| c(O2)7 |
| c(CO2)8 |
| c(O2)7 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
| ||
| ||
| ||
| ||
 Cr2O72-+H2O
Cr2O72-+H2O Cr2O72-+H2O
Cr2O72-+H2O�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ���� |
| ���� |
| ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�췯�ƣ��ظ����ƣ�Na2Cr2O7��2H2O������Ҫ�Ļ�������ԭ�ϡ���������̬��+3��+6�ۡ�������Ҫ��Ȼ��Դ�Ǹ�����FeCr2O4������Al2O3��MgO��SiO2�����ʣ���ʵ����ģ�ҵ�Ը�����Ϊԭ�������췯�Ƶ���Ҫ�������£�
������Ҫ��Ӧ��4 FeCr2O4 + 8Na2CO3+ 7O2 8Na2CrO4 + 2Fe2O3+ 8CO2
���и���Ӧ��Al2O3 + Na2CO3 2NaAlO2 + CO2����SiO2 + Na2CO3
Na2SiO3 + CO2��
��ش��������⣺
��1��FeCr2O4��ѧ��Ϊ�Ǹ���������д��������������ʽ________________�����衰�١��ķ�Ӧ������Ӧѡ��__________������ʡ��������ʡ���ʯӢ�ʡ�����
��2�����ڡ�������1�ijɷ���__________�����ۡ��е�pHֵ��_______ ������ߡ����͡��������ܡ�������2�ijɷ���___________��
��3�����ݡ��ữ��Ŀ����ʹCrO42��ת��ΪCr2O72����д��ƽ��ת�������ӷ���ʽ��__________________________��
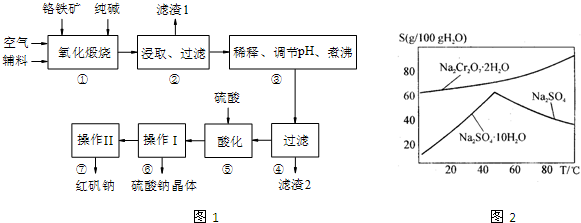
��4����ͼ��Na2Cr2O7��2H2O��Na2SO4���ܽ�����ߣ������I��______������II��______������ţ���
������Ũ�������ȹ��ˢڽ��½ᾧ������
��5����֪ij������Ԫ��34%�����в����~������ʧ2%�������~���в���Ϊ92%����1�ָÿ�ʯ�����Ͽ������췯��_______�֣�����2λС������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010���Ĵ������߿�Ԥ�⣨�ۺ��⣩��ѧ�� ���ͣ�ʵ����
��16�֣���������ʵ�������ʵ��������ȷ���ǣ� ��
A.���ù��˵ķ�����ȥ���������������������Ȼ���
B.����ʽ�ζ�����ȡ18.80mL��̼������Һ
C.����0.1mol/L������ʱ������Ͳ��ȡŨ���ᣬҪϴ����Ͳ���������Ƶ���ҺŨ��ƫ��
D.���ñ��͵�̼������Һ���������������Թܣ�����������ð��������CO2����
E.ʵ������ȡ����ʱ��������ˮ�Ȼ��ƽ��и���
���췯�ƣ��ظ����ƣ�Na2Cr2O2��2H2O������Ҫ�Ļ�������ԭ�ϣ���ӡȾ��ҵ����ƹ�ҵ��Ƥ�﹤ҵ�����������ڻ�ѧ��ҵ����ҩ��ҵ��������������Ӧ��ʮ�ֹ㷺��
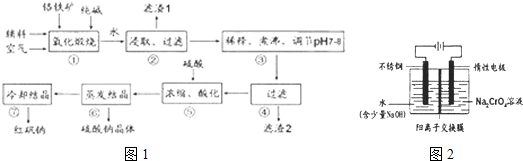
��1��ʵ�����к췯�ƿ��ø�������Ҫ�ɷ֣�FeO��Cr2O3����ԭ���������¹�������ȡ��
�ٲ���I�з�Ӧ�Ļ�ѧ����ʽΪ��
4FeO��Cr2O3(s)��8Na2CO3(s)��7O2 8Na2CrO4(s)��2Fe2O3(s)��8CO2���÷�Ӧ�Ļ�ѧƽ�ⳣ���ı���ʽΪ)_____________���ڳ����¸÷�Ӧ���ʼ��������д�ʩ�в���ʹ��Ӧ�����������____________ ��
8Na2CrO4(s)��2Fe2O3(s)��8CO2���÷�Ӧ�Ļ�ѧƽ�ⳣ���ı���ʽΪ)_____________���ڳ����¸÷�Ӧ���ʼ��������д�ʩ�в���ʹ��Ӧ�����������____________ ��
A�������¶� B����ԭ�Ϸ��� C�����Ӵ�������� D��ͨ������Ŀ���
�ڲ������������Һ�Լ��ԣ����г�����Na2CrO4�⣬������������Ԫ�صĻ�������ǵĻ�ѧʽ������__________��
�۲�����轫��Һ��pH����7��8����У���Ŀ����_________________________________��
�ܲ�����з�����Ӧ�����ӷ���ʽΪ______________________________________________��
(2�����췯����KCl���尴1��2�����ʵ���֮�ȣ��������ˮ���ʵ������ɵõ�K2Cr2O7���塣����д���пո������������
| ��� | ʵ�鲽�� | ����ʵ�����(�������������װʵ��װ��) |
| �� | �ܽ� | �����������ձ��С���ˮ����ֽ���ֱ�����岻���ܽ� |
| �� | | |
| �� | | |
| �� | | |
| �� | ���ˡ����� | �õ�K2Cr2O7���� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com