
����ʵ���ʧ��ԭ���������Ϊȱ�ٱ�Ҫ��ʵ�鲽����ɵ���
�ٽ��Ҵ�������ϡ���Ṳ������������
����ˮ�Ҵ���Ũ���Ṳ�ȵ�140������ϩ
����֤RX�ǵ���飬��RX���ռ���Һ��ϼ��Ⱥ���Һ��ȴ���ټ�����������Һ���ֺ�ɫ����
����ȩ�Ļ�ԭ��ʵ��ʱ�����������Ƶ�������ͭ����Һ��δ���ֺ�ɫ����
�ݼ��������ˮ�⣬������������ϡ�������һ��ʱ�����������Һˮԡ���Ⱥ�δ��������
A���٢ۢܢ� B���ۢܢ� C���ۢ� D��ȫ��
 ����ͬ�����Ծ�ϵ�д�
����ͬ�����Ծ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ijʵ��С�����ѧ�α��п����������ķ�Ӧ�������о����ܽ��������������H2�ķ�Ӧ����Zn+�����Na+ˮ����Al+NaOH��Һ��Ϊ��ȼ����������Ӧ���ɵ�H2�������������ͼ��ʾ��װ��ͼ��
ijʵ��С�����ѧ�α��п����������ķ�Ӧ�������о����ܽ��������������H2�ķ�Ӧ����Zn+�����Na+ˮ����Al+NaOH��Һ��Ϊ��ȼ����������Ӧ���ɵ�H2�������������ͼ��ʾ��װ��ͼ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ijʵ��С�����ѧ�α��п����������ķ�Ӧ�������о����ܽ��������������H2�ķ�Ӧ����Zn�����ᡡ��Na��ˮ����Al��NaOH��Һ��Ϊ��ȼ����������Ӧ���ɵ�H2���������������װ��(ͼ1)����ش��������⣺
(1)д��Na��H2O��Ӧ�Ļ�ѧ����ʽ_____________________________________��
(2)�ڵ�ȼH2ǰ�����Ƚ���_____________________________________________��
������ _______________________________________________________��
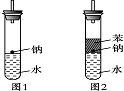 (3)ʵ��С���ڵ�ȼ��ͼ1װ���Ƶõ�H2ʱ���٢�ʵ���óɹ�����ȴʧ���ˡ����Ƿ�����Ϊʧ�ܵ�ԭ����Na��H2O�ķ�Ӧ����̫�죬Na������̫�١����������������Ƶ�����������ʦ˵̫Σ�գ�����Ϊ����Σ�յ�ԭ����________________________________________________________________________��
(3)ʵ��С���ڵ�ȼ��ͼ1װ���Ƶõ�H2ʱ���٢�ʵ���óɹ�����ȴʧ���ˡ����Ƿ�����Ϊʧ�ܵ�ԭ����Na��H2O�ķ�Ӧ����̫�죬Na������̫�١����������������Ƶ�����������ʦ˵̫Σ�գ�����Ϊ����Σ�յ�ԭ����________________________________________________________________________��
(4)ʵ��С������ơ�����ˮ���ܶȷֱ�Ϊ0.97 g��mL��1��0.88 g��mL��1��1.00 g��mL��1�����ݴ˶�ʵ������˸Ľ�(��ͼ2)���ڸĽ����ʵ����H2���������ʼ�����ԭ����________________________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013��ɽ��ʡ���¶��и߿�һ�ָ�ϰ�ο��Ի�ѧ�Ծ����������� ���ͣ�ʵ����
ijʵ��С�����ѧ�α��п����������ķ�Ӧ�������о����ܽ���ĸ���������H2�ķ�Ӧ����Zn+�����Na+ˮ ��Al+NaOH��Һ��Na+��ˮ�Ҵ���Ϊ��ȼ�����ĸ���Ӧ���ɵ�H2���������������װ��ͼ��
��ش��������⣺
��д��Na��H2O��Ӧ�Ļ�ѧ����ʽ ��
���ڵ�ȼH2֮ǰ�����Ƚ��� ��������
��
��ʵ��С���ڵ�ȼ������װ���Ƶõ�H2ʱ���٢ۢ�ʵ���óɹ�����ȴ
ʧ���ˡ����Ƿ�����Ϊʧ�ܵ�ԭ����Na��H2O�ķ�Ӧ����̫�죬Na��
����̫�١����������������Ƶ�����������ʦ˵̫Σ�գ�����Ϊ����Σ
�յ�ԭ���� ��
��ʵ��С������ơ�����ˮ���ܶȷֱ�Ϊ0.97g/mL��0.88g/mL��1.00g/mL�����ݴ˶�ʵ������˸Ľ���
�ڸĽ����ʵ����H2���������ʼ�����ԭ����
��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ��ɽ��ʡ�߿�һ�ָ�ϰ�ο��Ի�ѧ�Ծ��������棩 ���ͣ�ʵ����
ijʵ��С�����ѧ�α��п����������ķ�Ӧ�������о����ܽ���ĸ���������H2�ķ�Ӧ����Zn+���� ��Na+ˮ ��Al+NaOH��Һ ��Na+��ˮ�Ҵ���Ϊ��ȼ�����ĸ���Ӧ���ɵ�H2���������������װ��ͼ��

��ش��������⣺
��д��Na��H2O��Ӧ�Ļ�ѧ����ʽ ��
���ڵ�ȼH2֮ǰ�����Ƚ��� ��������
��
��ʵ��С���ڵ�ȼ������װ���Ƶõ�H2ʱ���٢ۢ�ʵ���óɹ�����ȴ
ʧ���ˡ����Ƿ�����Ϊʧ�ܵ�ԭ����Na��H2O�ķ�Ӧ����̫�죬Na��
����̫�١����������������Ƶ�����������ʦ˵̫Σ�գ�����Ϊ����Σ
�յ�ԭ���� ��
��ʵ��С������ơ�����ˮ���ܶȷֱ�Ϊ0.97g/mL��0.88g/mL��1.00g/mL�����ݴ˶�ʵ������˸Ľ���
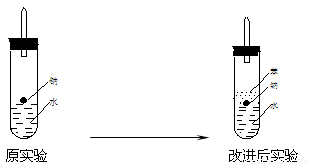
�ڸĽ����ʵ����H2���������ʼ�����ԭ����
��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ�갲�պϷʰ��и������Ĵ��¿���ѧ�Ծ� ���ͣ�ʵ����
��12�֣�����ҩ������ơ�˵���鲿������ժ¼��
�����ÿƬ������������0.1g
���������״���ں�Fe2+34.0%��36.0%����ˮ��ʽ�Σ�Ϊ��Ĥ����Ƭ
����Ӧ֢������ȱ����ƶѪ֢Ԥ��������
�������÷�������Ԥ����0.1g���գ�������0.2g��0.4g���գ�С��Ԥ����30��60mg���գ�������0.1g��0.3g����
�����ء��ڱܹ⡢�ܷ⡢����������
��ҩ������á���ά����Cͬ���������ӱ�Ʒ���գ���ҩƬ��ˮ���Ҵ����ܽ�Ȳ���
��ijѧУ��ѧʵ��С�飬Ϊ�˼�⡰�����ơ�ҩƬ������Ԫ�صĴ��ڣ���������¼���ʵ�飺
��������Ʒ�����
 ����ͬѧ������Ƶķ������ʵ�飬���ź���������û�еõ�Ԥ�ڵ�ʵ������
����ͬѧ������Ƶķ������ʵ�飬���ź���������û�еõ�Ԥ�ڵ�ʵ������
��1������ʵ��ʧ�ܵĿ���ԭ��________________________________________________ ��
����ͬѧ����˼���˼���ʵ��ʧ�ܵ�ԭ��ģ��ҩƬ���ú����������ܽ�ı仯���̣�������Ʋ��������ʵ�飺

��2�����Լ�1Ϊ������Լ�2Ϊ________________________________��
����ͬѧ������ʵ��������ɫ��ȥ������������о���Ȥ��̽����ɫ��ԭ����������ɣ�������Ϊ�����ֿ��ܵ�ԭ��
[Fe��SCN��]2+�������е�����������ԭΪ����
�� ��
��3�����������һ�ֿ��ܽ���ʵ����֤��________________________________
ʵ�鷽����������������________________________________________________ ,
����Ԥ�������жϽ��ۣ�________________________________________________________ ��
��4�������������ơ�1.0 g������ȫ������ϡ�����У����Ƴ�100.00 mL��Һ��ȡ��20.00 mL����0.01000 mol/L��KMnO4��Һ�ζ������β����������£�
|
��� |
V��KMnO4���� |
V��KMnO4���� |
V��KMnO4�� |
|
1 |
2.24mL |
14.25mL |
12.01mL |
|
2 |
0.30mL |
12.72mL |
12.42mL |
|
3 |
0.50mL |
12.53 |
12.03mL |
���㣺�ò�Ѫҩ�к�Fe2+����������________________������С������λС������
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com