
 ��һ�������£�����ؿ��к������ķǽ���Ԫ��X��������ֳ����Ļ������X��O�����ֻ���������еĻ�ѧ��Ϊ���ۼ�
��һ�������£�����ؿ��к������ķǽ���Ԫ��X��������ֳ����Ļ������X��O�����ֻ���������еĻ�ѧ��Ϊ���ۼ�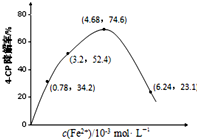
���� ��1��Sԭ�Ӻ�����16�����ӣ�������16�����ӣ��������Ų����ؿ��к������ķǽ���Ԫ��ΪO��O��S�γɹ��ۼ���
��2�����ݻ��ϼ��ж������������������Ϸ���ʽ���㣻SO2��FeCl3��Һ��Ӧ����Fe2+��SO42-��
��3������ԭ���غ�ɵó���ѧʽ��
��4���ٵ�����������ͬ����4-CP���뵽��ͬpH��Na2S2O8��Һ�У�����ͼ��֪����Һ��pHԽС��4-CP������Խ��
��A��������Һ��pH��4-CP�Ľ����ʿ���֪����Һ������ǿ��������Na2S2O8����SO4-��
B�����Ի�����4-CP���ⷴӦ�Ĵ�����
C��Fe2+����SO4-������Ӧ�����IJ���SO4-��
D�����ɵ�Fe3+ˮ��ʹ��Һ��������ǿ�ʣ�
�۸���ͼƬ֪����c��Fe2+��=3.2��10-3mol•L-1ʱ��ȷ��4-CP�����ʶ�Ӧλ�ã�ƽ����Ӧ����=$\frac{��c}{��t}$��
��� �⣺��1��Sԭ�Ӻ�����16�����ӣ�������16�����ӣ��������Ų�����ԭ�ӽṹʾ��ͼΪ ���ؿ��к������ķǽ���Ԫ��ΪO��O��S�γɶ����������������S��O֮���γɹ��ۼ���
���ؿ��к������ķǽ���Ԫ��ΪO��O��S�γɶ����������������S��O֮���γɹ��ۼ���
�ʴ�Ϊ�� ��O�����ۼ���
��O�����ۼ���
��2����Ӧ4FeS2+11O2 $\frac{\underline{\;����\;}}{\;}$2Fe2O3+8SO2��ǰ���Ԫ�ػ��ϼ۵ı仯������£�
Fe��+2��+3�����ϼ����ߣ�S��-1��+4�����ϼ����ߣ�
O��0��-2�����ϼ۽��ͣ�
��ˣ��ڷ�Ӧ��FeS2��ԭ����O2����������Fe2O3������������Ҳ�ǻ�ԭ���SO2������������Ҳ�ǻ�ԭ���n������������n����ԭ���=11��10��
SO2��FeCl3��Һ��Ӧ����Fe2+��SO42-���䷴Ӧ�����ӷ���ʽΪ��SO2+2Fe3++2H2O=2Fe2++SO42-+4H+��
�ʴ�Ϊ��11��10��SO2+2Fe3++2H2O=2Fe2++SO42-+4H+��
��3��1mol Na2S2O8�ֽ�����1mol�������ƣ������������11.2L O2�����ʵ���Ϊ0.5mol������ԭ��Ϊ1mol��˵��1mol������������ԭ��Ϊ7mol���������ƵĻ�ѧʽΪ��
Na2S2O7���ʴ�Ϊ��Na2S2O7��
��4���ٵ�����������ͬ����4-CP���뵽��ͬpH��Na2S2O8��Һ�У�����ͼ��֪����Һ��pHԽС��4-CP������Խ��������Һ������ǿԽ������Na2S2O8����SO4-��
�ʴ�Ϊ��Խ�ã�
��A��������Һ��pH��4-CP�Ľ����ʿ���֪����Һ������ǿ��������Na2S2O8����SO4-����A��ȷ��
B�����Ի�����4-CP���ⷴӦ�Ĵ�������B����
C��Fe2+����SO4-������Ӧ�����IJ���SO4-������c��Fe2+������ʱ��4-CP�����ʷ����½�����C��ȷ��
D�����ɵ�Fe3+ˮ��ʹ��Һ��������ǿ�������������4-CP�Ľ��⣬��D����
��ѡAC��
�۸���ͼƬ֪����c��Fe2+��=3.2��10-3mol•L-1ʱ��4-CP������Ϊ52.4��4-CP�����ƽ����Ӧ����=$\frac{1.56��1{0}^{-4}mol/L��52.4%}{240min}$=$\frac{1.56��1{0}^{-4}��52.4%}{240}$mol/��L/min����
�ʴ�Ϊ��52.4��$\frac{1.56��1{0}^{-4}��52.4%}{240}$mol/��L/min����
���� ���⿼�������仯��������ʣ��漰ԭ�ӽṹʾ��ͼ�����ӷ���ʽ����ȷ�����й�֪ʶ����Ŀ�ۺ���ǿ�����ؿ���ѧ����ȡ��Ϣ���ӹ���Ϣ��������Ϣ�������������֪��ͼ�����ݺ�����ĺ��弰�仯���ƣ�֪��ʵ��Ŀ�ļ�ԭ������Ŀ�ѶȲ���



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ������$\frac{c��{H}^{+}��}{c��{OH}^{-}��}$=1012����Һ�У�Fe2+��Mg2+��NO3-��Cl- | |
| B�� | ���д���Al3+����Һ�У�Na+��NH4+��Cl-��HCO3- | |
| C�� | ��ʹpH��ֽ����ɫ����Һ�У�K+��Ba2+��Cl-��Br- | |
| D�� | �����£���ˮ�������c��H+��=1��10-13 mol•L-1����Һ�У�K+��Fe3+��CH3COO-��SO42-��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ���ƹܵ�Ϳ���ۿɷ���ʴ | |
| B�� | ̼�ᱵ��̼�����ơ�������þ�Ⱦ�����Ϊ����ҩ��ʹ�� | |
| C�� | �����м�Ǧ��Ŀ��������۵� | |
| D�� | �÷Ͼ�Ƥ������ҩ�ý��ҿ������ԭ�������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��״���£�22.4LH2O���еķ�����Ϊ1 NA | |
| B�� | ���³�ѹ�£�16g O2���е�ԭ����Ϊ1 NA | |
| C�� | ͨ��״���£�1 NA ��SO2����ռ�е����Ϊ22.4L | |
| D�� | ���ʵ���Ũ��Ϊ0.5mol/L��K2CO3��Һ�У�����CO32-����Ϊ0.5 NA |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� |  | B�� |  | C�� |  | D�� |  |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com