
������һ�������Դ����������ȡ�봢��������Դ����������о��ȵ㡣
��֪�� ��g��+
��g��+  ��g��====
��g��====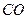 ��g��+
��g��+ ��g��
��g��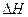 =
= 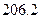
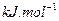
 ��g��+
��g��+  ��g��====
��g��====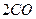 ��g��+
��g��+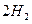 ��g��
��g��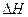 =
= 
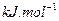
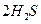 ��g��====
��g��====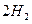 ��g��+
��g��+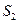 ��g��
��g�� 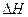 =
= 
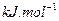
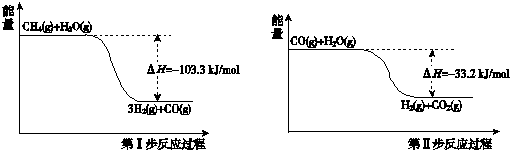
��1���Լ���Ϊԭ����ȡ�����ǹ�ҵ�ϳ��õ����ⷽ���� ��g����
��g���� ��g����Ӧ����
��g����Ӧ���� ��g����
��g���� ��g�����Ȼ�ѧ����ʽΪ______��
��g�����Ȼ�ѧ����ʽΪ______��
��2��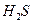 �ȷֽ�����ʱ������Ӧ����ͨ��һ������������ʹ����
�ȷֽ�����ʱ������Ӧ����ͨ��һ������������ʹ����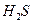 ȼ�գ���Ŀ����_____;ȼ�����ɵ�
ȼ�գ���Ŀ����_____;ȼ�����ɵ�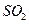 ��
��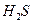 ��һ����Ӧ���������ڳ����¾������壬д���÷�Ӧ�Ļ�ѧ����ʽ��_______��
��һ����Ӧ���������ڳ����¾������壬д���÷�Ӧ�Ļ�ѧ����ʽ��_______��
��3��H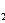 O���ȷֽ�Ҳ�ɵõ�H
O���ȷֽ�Ҳ�ɵõ�H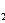 ��������ˮ�ֽ���ϵ����Ҫ���������������¶ȵĹ�ϵ��ͼ11��ʾ��ͼ��A��B��ʾ������������_______��
��������ˮ�ֽ���ϵ����Ҫ���������������¶ȵĹ�ϵ��ͼ11��ʾ��ͼ��A��B��ʾ������������_______��
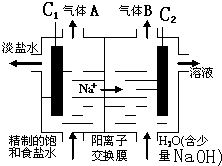
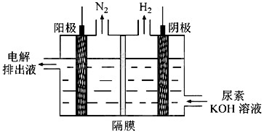
��4���������[CO(NH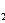 )
)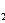 ]�ļ�����Һ�����װ��ʾ��ͼ��ͼ12�������и�Ĥ����ֹ����ͨ��������������Ϊ���Ե缫�������ʱ�������ĵ缫��ӦʽΪ_______��
]�ļ�����Һ�����װ��ʾ��ͼ��ͼ12�������и�Ĥ����ֹ����ͨ��������������Ϊ���Ե缫�������ʱ�������ĵ缫��ӦʽΪ_______��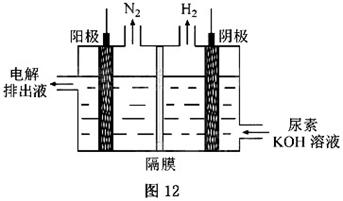
��5��Mg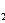 Cu��һ�ִ���Ͻ�350��ʱ��Mg
Cu��һ�ִ���Ͻ�350��ʱ��Mg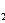 Cu��H
Cu��H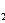 ��Ӧ������MgCu
��Ӧ������MgCu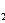 �ͽ���һ�ֽ���Ԫ�ص��⻯����������������Ϊ0.077����Mg
�ͽ���һ�ֽ���Ԫ�ص��⻯����������������Ϊ0.077����Mg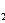 Cu��H
Cu��H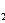 ��Ӧ�Ļ�ѧ����ʽΪ_______��
��Ӧ�Ļ�ѧ����ʽΪ_______��
 ��У����ϵ�д�
��У����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ������һ�������Դ������ͨ�����ַ����Ƶã�
������һ�������Դ������ͨ�����ַ����Ƶã�| c(CO)c(H2) |
| c(H2O) |
| c(CO)c(H2) |
| c(H2O) |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ������һ�������Դ����������ȡ�봢��������Դ����������о��ȵ㣮
������һ�������Դ����������ȡ�봢��������Դ����������о��ȵ㣮�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
 ������һ�������Դ����������ȡ�봢��������Դ����������о��ȵ㣮
������һ�������Դ����������ȡ�봢��������Դ����������о��ȵ㣮| 1 |
| 2 |
c(H2)c
| ||
| c(H2O) |
c(H2)c
| ||
| c(H2O) |
| 1 |
| 2 |
| 1 |
| 2 |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com