
(6��)
�ҹ�����Ϊ��������ȱ�������涨��ʳ���б�����������ĵ���ء�����ʳ�����Ƿ�ӵ⣬���������·�Ӧ��KIO3+5KI+3H2 SO4====3K2SO4+3I2+3H2O
SO4====3K2SO4+3I2+3H2O
(1)�á�˫���š���ʾ��������Ӧ�е���ת�Ƶķ������Ŀ
________________________________________��
(2)�����Ӧ��ת��0��2mol���ӣ�������I2�����ʵ���Ϊ_________________��
(3)����������Ӧ����ʳ �����Ƿ�ӵ⣬�����Լ���______________(������ѡ������)
�����Ƿ�ӵ⣬�����Լ���______________(������ѡ������)
�ٵ�ˮ ��KI��Һ �۵�����Һ ��ϡ���� ��AgNO3��Һ
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(6��)�ҹ�����Ϊ��������ȱ�������涨��ʳ���б�����������ĵ���ء�����ʳ�����Ƿ�ӵ⣬���������·�Ӧ��KIO3+5KI+3H2SO4====3K2SO4+3I2+3H2O
(1)�á�˫���š���ʾ��������Ӧ�е���ת�Ƶķ������Ŀ
________________________________________��
(2)�����Ӧ��ת��0��2mol���ӣ�������I2�����ʵ���Ϊ_________________��
(3)����������Ӧ����ʳ�����Ƿ�ӵ⣬�����Լ���______________(������ѡ������)
![]() �ٵ�ˮ ��KI��Һ �۵�����Һ ��ϡ���� ��AgNO3��Һ
�ٵ�ˮ ��KI��Һ �۵�����Һ ��ϡ���� ��AgNO3��Һ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014�����ʡ�����и�һ��ѧ�����п��Ի�ѧ�Ծ� ���ͣ������
(6��)�ҹ�����Ϊ��������ȱ�������涨��ʳ���б�����������ĵ���ء�����ʳ�����Ƿ�ӵ⣬���������·�Ӧ��KIO3+5KI+3H2SO4====3K2SO4+3I2+3H2O
(1)�á�˫���š���ʾ��������Ӧ�е���ת�Ƶķ������Ŀ
________________________________________��
(2)�����Ӧ��ת��0��2mol���ӣ�������I2�����ʵ���Ϊ_________________��
(3)����������Ӧ����ʳ�����Ƿ�ӵ⣬�����Լ���______________(������ѡ������)
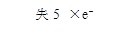 �ٵ�ˮ ��KI��Һ �۵�����Һ ��ϡ���� ��AgNO3��Һ
�ٵ�ˮ ��KI��Һ �۵�����Һ ��ϡ���� ��AgNO3��Һ
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com