
X��Y��Z��M��Q��G���ֶ�����Ԫ�أ�ԭ��������������X��Zͬ���壬���γ����ӻ�����ZX��Y��Mͬ���壬Y��һ��ͬλ��ԭ�ӳ����ڲⶨ����������Q�γɵĵ���Ϊ����ɫ���塣��ش��������⣨�漰���ʾ��û�ѧʽ��ʾ����
��1�����ӻ�����ZX��X���ӵĽṹʾ��ͼΪ �� Y��Ԫ�����ڱ��е�λ����_______________��
��2������Ԫ�ص�����������Ӧ��ˮ����������ǿ����_______________��Q��G����̬�⻯�ﻹԭ�Ը�ǿ����__________________��
��3����ҵ���Ʊ�M�ĸߴ��ȵ��ʣ�����һ����Ҫ��Ӧ�ǣ���MXG3��X2�ڸ����·�Ӧ���÷�Ӧ���̱��������ˮ��������ΪMXG3��ˮ���ҷ�Ӧ����H2�� �� ������������������ĺ���� ��
��4��X2Q��ȼ����Ϊa kJ·mol-1������X2Qȼ�շ�Ӧ���Ȼ�ѧ����ʽ��ȷ���� ��
| A��2X2Q(g) + O2(g) =" 2Q(s)" + 2X2O(g)��H=" -2a" kJ·mol-1 |
| B��X2Q(g) + 2O2(g) = QO3(g) + X2O(l)��H=" +a" kJ·mol-1 |
| C��2X2Q(g)+ 3O2(g) = 2QO2(g) + 2X2O(l)��H=" -2a" kJ·mol-1 |
| D��X2Q(g) + 2O2(g) = QO3(g) + X2O(l)��H=" -a" kJ·mol-1 |
 Fe + 2ZG �ŵ�ʱ����ص�������ӦʽΪ: �õ�صĵ����Ϊ___________________��
Fe + 2ZG �ŵ�ʱ����ص�������ӦʽΪ: �õ�صĵ����Ϊ___________________��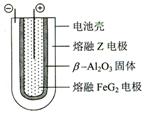
��16�֣���1�� ��2�֣� �ڶ�����IVA�壨2�֣� ��2��HClO4��1��H2S��1�֣�
��2�֣� �ڶ�����IVA�壨2�֣� ��2��HClO4��1��H2S��1�֣�
��3��H2SiO3��1�֣���HCl��1�֣���H2��O2��Ϸ�����ը�����ɵ�Si���ֱ�O2������SiO2��1�֣�
��4��C ��3�֣� ��5��Fe2+ +2e- =" Fe" ��2�֣��� ��-Al2O3 ��2�֣�
���������������������Ԫ�ض��Ƕ�����Ԫ�أ����Ը�����֪�����ƣ�Q����Ϊ����ɫ���壬��Q��S����S֮��Ķ���������Ԫ����Cl����GΪCl������ͬλ�زⶨ��ΪC��YΪCԪ�أ���Cͬ�����MӦ����Si����CԪ��֮ǰ��XԪ����ͬ����Ԫ��Z�γ����ӻ�����ZX����X��Z���ڵ�һ���壬�ҷֱ�ΪH��Na���������Ͽɵ�X��Y��Z��M��Q��GԪ�طֱ���H��C��Na��Si��S��Cl��
��1��X��HԪ�أ�������NaH�У�H-�����ӽṹʾ��ͼΪ ��YΪCԪ�أ���λ���ǣ��ڶ����ڣ���IVA�塣
��YΪCԪ�أ���λ���ǣ��ڶ����ڣ���IVA�塣
��2������6��Ԫ���зǽ�����ǿ����Cl�������������Ӧ��ˮ����HClO4������ǿ��Q��G���⻯��ֱ�ΪH2S��HCl����Ϊ�ǽ�����Cl>S�����ʵ�������Cl2>S���������ӵĻ�ԭ��Cl-<S2- �����Ի�ԭ��ǿ���⻯����H2S��
��3��MXG3��X2 �ֱ�ΪSiHCl3��H2���������ö����Ʊ��ߴ���ʱ����������ˮSiHCl3����H2O��������ˮ�ⷴӦ�����ɹ���H2��H2SiO3��HCl���������������������¼��Ȳ���H2�ᷢ����ը������O2�ֻ������������ɵĵ��ʹ裬����Ҫ������ˮ����������
��4��X2Q��H2S��H2Sȼ����һ�����ȷ�Ӧ�����ԡ�H<0��ȼ����a kJ·mol-1��ָ1mol��������ȫȼ�������ȶ�������SO2ʱ���ͷŵ������������������ʽϵ����2ʱ����Ӧ���ʱ��H=" -2a" kJ·mol-1 ������Ӧ��ѡ��C��
��5���õ�ط�ӦʽΪ��2Na+FeCl2 Fe+2NaCl�����Կ�֪�÷�Ӧ�зŵ�ʱ����Na����ظ���ʧȥ���ӱ�������Fe2+�������õ����ӱ���ԭΪFe���ʣ�����������ӦʽΪ��Fe2+ + 2e- =Fe�����ڵ�ط�Ӧʽ������״̬�½��еģ����Դ�ͼ������жϳ��õ�صĵ������Һ�Ǧ�-Al2O3��
Fe+2NaCl�����Կ�֪�÷�Ӧ�зŵ�ʱ����Na����ظ���ʧȥ���ӱ�������Fe2+�������õ����ӱ���ԭΪFe���ʣ�����������ӦʽΪ��Fe2+ + 2e- =Fe�����ڵ�ط�Ӧʽ������״̬�½��еģ����Դ�ͼ������жϳ��õ�صĵ������Һ�Ǧ�-Al2O3��
���㣺���⿼�����Ԫ�����ڱ���Ԫ�������ɡ��ǽ������仯�������ʡ���ѧ��Ӧ���������绯ѧ������֪ʶ��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
X��Y��Z��W��Ԫ�����ڱ�ǰ�������еij���Ԫ�أ��������Ϣ���±���
| Ԫ�� | �� �� �� Ϣ |
| X | X����������ˮ��������̬�⻯������γ�һ���� |
| Y | ���������õİ뵼����ϣ��㷺Ӧ���ڹ����Ϣ���� |
| Z | Z��һ�ֺ���������Ϊ27��������Ϊ14 |
| W | ����������Ӧ��ˮ������һ�ֲ�����ˮ����ɫ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
a��b��c��d��e��f��gΪ�����ɶ�������Ԫ�ع��ɵ����ӣ����Ƕ���10�����ӣ���ṹ�ص����£�
| ���Ӵ��� | a | b | c | d | e | f | g |
| ԭ�Ӻ��� | ���� | ���� | ˫�� | ��� | ���� | ��� | ��� |
| ���������λ��ɣ� | 0 | 1+ | 1- | 0 | 2+ | 1+ | 0 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
���в��ֶ�����Ԫ�ص����ʻ�ԭ�ӽṹ���±���
| Ԫ�ر�� | Ԫ�����ʻ�ԭ�ӽṹ |
| T | ��������ˮ���ҷ�Ӧ������ǿ������Һ�к������ֵ�������ͬ������������ |
| X | �������������ڲ��������3�� |
| Y | ��������Ԫ�صļ������а뾶��С |
| Z | T��X��Z��ɵ�36���ӵĻ�����A�Ǽ�������������Ҫ�ɷ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
X��Y��Z��W���ֳ���Ԫ�أ�����X��Y��ZΪ������Ԫ�ء��й���Ϣ���±���
| | ԭ�ӻ���������Ϣ | ���ʼ��仯���������Ϣ |
| X | ZX4�������ɴ�Z�ᴿZ���м���� | X������������Ӧ��ˮ����Ϊ��������ǿ�� |
| Y | Yԭ�ӵ��������������ڵ��Ӳ��� | Y���������ǵ��͵��������������������һ�ּ���ǰ;�ĸ��²��� |
| Z | Zԭ�ӵ������������Ǵ�����������1/2 | Z�����ǽ������ϵ����ǣ��䵥������ȡ���ģ���ɵ�·����Ҫԭ�� |
| W | Wԭ�ӵ�����������С��4 | W�ij������ϼ���+3��+2��WX3ϡ��Һ�ʻ�ɫ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ͼΪ�ġ��塢����������Ԫ�ؼ��⻯��е������ڱ仯ͼ�������⻯A��B��C��D��������һ��Ԫ�أ�����ͼ�ش�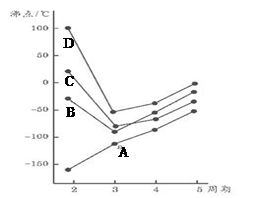
��1��д��B3���Ŀռ乹��
��2��BԪ������һ���⻯����������ȼ�ϣ������ʽΪ��__________________������ԭ���ӻ���ʽΪ_______________
��3��AԪ�������ᄃ������Ϊ____________________���о������������ᄃ���У�A��λ������ʯ������̼ԭ��λ�����ƣ��Է���������ռ����ԭ�Ӹ���ӦΪ___________����
��4��A��B��C��D��һ��������С�����˳����____________________����Ԫ�ط��ű�ʾ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��11�֣���1������A��B��C��D��E����ԭ����������������Ԫ�أ�A��ԭ�Ӻ���û�����ӣ�B��DԪ�ش���ͬһ���ڣ�D��ԭ�Ӱ뾶��С��B��D��������֮��Ϊ27��������֮��Ϊ5��C�ĵ��ʸ����ᷴӦ������C3����������E��D���γ�E2D�����ӻ������E��D��Ԫ�صļ����Ӿ�����ͬ���Ӳ�ṹ��
�� д��Ԫ�ط��ţ�A ��D ��E ��
�� B��C������������Ӧˮ�������Ӧ�����ӷ���ʽΪ��
��
�� д��A2D�ĵ��뷽��ʽ�� �� ��
��2��Ԫ�������ڱ��е�λ�ã���ӳ��Ԫ�ص�ԭ�ӽṹ��Ԫ�ص����ʡ�N P AS ;O S Se�ֱ���VA�塢VIA��������Ԫ�ء�
�ٸ���Ԫ�������ɣ�Ԥ�⣺����ǿ�� H3AsO4 H3PO4�����á�>��������ʾ��
�� Ԫ��S��������ۺ�����۵Ĵ�����Ϊ____________����һ�������£�S��H2��Ӧ��һ���ȣ����жϣ�����ͬ������Se��H2��Ӧ���ȱ�S��H2��Ӧ�� ��(ѡ���������С������ͬ��)
�۽�SO2����ͨ����ˮǡ����ȫ��Ӧ����Һ�д��ڵ�����Ũ���ɴ�С��˳����_________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
A��B��C��D����4��Ԫ�أ��������������
��1��AԪ�صĻ�̬ԭ�ӵ��������3��δ�ɶԵ��ӣ��������2�����ӣ�AԪ�ص������� ����Ԫ�ص�ԭ�Ӻ���� �ֲ�ͬ�˶�״̬�ĵ��ӣ���Ԫ�صĵ�һ�����ܱȺ���һ��Ԫ�ش��ԭ���� ��
��2��BΪԪ�����ڱ��е�29��Ԫ�أ�����H2O��NH3���γ�����
B����ˮ�����ξ���� ɫ����ʢ��B��������ˮ��Һ���Թ�����μ��백ˮ�������γ� ���������Ӱ�ˮ�������ܽ�õ� ��д����һ����Ӧ�����ӷ���ʽ ��
��3��CԪ�صĸ�һ�����ӵĵ��Ӳ�ṹ�����ͬ��C������������ˮ����Ļ�ѧʽΪ ��
��4��AԪ�ص�����������Ӧ��ˮ�����ϡ��Һ��29��Ԫ�صĵ��ʷ�Ӧ�����ӷ���ʽ��____________________��
��5��DԪ����Ԫ�����ڱ��ĸ�Ԫ���У��縺�Խ�С�ڷ���A����ͼ��⻯����D��һ���⻯�ﷴӦ�����ɵ����ֲ��������Ⱦ�������䷴Ӧ�Ļ�ѧ����ʽΪ___________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
A��B��C��D��E��F��Ԫ�����ڱ�ǰ�����ڵ�����Ԫ�أ�ԭ���������������������Ϣ���±���
| Ԫ�� | �����Ϣ |
| A | ԭ�ӵĺ���������͵��Ӳ������ |
| B | ��̬ԭ�Ӽ۵����Ų�Ϊnsnnpn |
| C | ��̬�⻯��������������ˮ���ﷴӦ������ |
| D | ��̬ԭ�ӵ����Ų���2��δ�ɶԵ��� |
| E | λ�ڵ������ڣ���ͬ������ԭ�Ӱ뾶��� |
| F | ��B �γɵĺϽ�ΪĿǰ�������Ľ������� |
 2CA3��H��0����Ӧ10min�ﵽƽ�⣬����0.2molCA3��
2CA3��H��0����Ӧ10min�ﵽƽ�⣬����0.2molCA3���鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com