
ʵ���������һ�������£���ϩ��ˮ�ķ�Ӧ�ɱ�ʾΪ��
C2H4(g) + H2O (g) = C2H5OH(g) ��H = ��45.8 kJ/mol
������˵������ȷ���ǣ� ��
A��ʵ���У���ϩ����������Ӱ��÷�Ӧ�ķ�Ӧ�ʱ䦤H
B��0.5 mol H2O(l) ��ȫ��Ӧ�ų�������Ϊ22.9 kJ
C��1 mol C2H5OH(g)�����������1 mol C2H4(g)��1 mol H2O (g)�����������
H2O (g)�����������
D��1 mol C2H4(g)��1 mol H2O (g)�л�ѧ�����ܼ��ܴ���1 mol C2H5OH(g)�л�ѧ�����ܼ���
 ���ɶ���ܲ��¿�ֱͨ�п�ϵ�д�
���ɶ���ܲ��¿�ֱͨ�п�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ��ӱ�ʡ�߶���ѧ�ڵ�һ���¿���ѧ�Ծ��������棩 ���ͣ�ѡ����
NH3�ʹ�����O2��һ�������·�����Ӧ��4NH3(g)��3O2(g) 2N2(g)��6H2O(g)������һ�ݻ������2 L�ܱ������г���4 mol NH3��3 mol O2, 4 min������ɵ�H2Oռ������������40%�������б�ʾ�˶�ʱ���ڸ÷�Ӧ��ƽ�����ʲ���ȷ����( )
2N2(g)��6H2O(g)������һ�ݻ������2 L�ܱ������г���4 mol NH3��3 mol O2, 4 min������ɵ�H2Oռ������������40%�������б�ʾ�˶�ʱ���ڸ÷�Ӧ��ƽ�����ʲ���ȷ����( )
A��v(N2)��0.125 mol/(L��min) B��v(H2O)��0.375 mol/(L��min)
C��v(O2)��0.225 mol/(L��min) D��v(NH3)��0.250 mol/(L��min)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2017�����ʡ������ѧ��9���¿���ѧ�Ծ��������棩 ���ͣ�ѡ����
�����йػ�ѧ����ı�ʾ��ȷ����
A��Ne��Na���Ľṹʾ��ͼ��Ϊ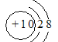
B��NaHCO3��ˮ�еĵ��뷽��ʽΪNaHCO3=Na++HCO3��
C��������ϩ�Ľṹ��ʽΪ
D��MgCl2�ĵ���ʽΪ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2017�콭��ʡ������ѧ�ڵ�һ�ζο���ѧ���������棩 ���ͣ�ѡ����
��֪ij������Һ�д��ڽ϶��Cu2+��NO3���������Һ�л����ܴ������ڵ���������
A��OH����CO32����Na+ B��SO42����Cl����NH4+
C��ClO����HCO3����K+ D��Br����Fe2+��Ba2+
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ��ӱ�ʡ�߶���ѧ�ڵ�һ���¿���ѧ�Ծ��������棩 ���ͣ������
��һ�ܱ������з�����ӦN2��3H2 2NH3 ��H��0���ﵽƽ���ֻ�ı�ijһ������ʱ����Ӧ������
2NH3 ��H��0���ﵽƽ���ֻ�ı�ijһ������ʱ����Ӧ������ ��Ӧʱ��Ĺ�ϵ��ͼ��ʾ��
��Ӧʱ��Ĺ�ϵ��ͼ��ʾ��
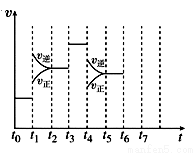
�ش��������⣺
��1�� t1��t3��t4ʱ�̷ֱ�ı��һ��������
A����ѹǿ B��Сѹǿ C�����¶�
D�����¶� E�Ӵ��� F���뵪��
��t1ʱ�� _��
��2������(1)�еĽ��ۣ�����ʱ����У����İٷֺ�����ߵ��� (��ѡ��)��
A��t0��t1 B��t2��t3 C��t3��t4 D��t5��t6
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ��ӱ�ʡ�߶���ѧ�ڵ�һ���¿���ѧ�Ծ��������棩 ���ͣ������
��Ӧ3Fe��s��+4H2O��g��====Fe3O4��s��+4H2��g������һ�ɱ���ݻ����ܱ������н��У��Իش�
��1����Fe������������Ӧ���ʵı仯��__________���������䡢��С��������ͬ��
��2���������������Сһ�룬������Ӧ����__________��ƽ��___________�ƶ�������ƶ���������Ӧ�������淴Ӧ�����ƶ���������ͬ��
��3������������䣬����N2ʹ��ϵѹǿ����������Ӧ����___________��ƽ��___________�ƶ���
��4������ѹǿ���䣬����N2ʹ�������������������Ӧ����_____________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ��ӱ�ʡ�߶���ѧ�ڵ�һ���¿���ѧ�Ծ��������棩 ���ͣ������
2.00gC2H2ȫȼ������Һ̬ˮ��CO2���ų�99.6kJ������3.00molC2H2��ȫȼ���ܷų� ������ȼ����ͬ���ʵ�CH4��C2H2�� ����ȼ�շų��������ࣿ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ�꼪��ʡ�߶���ѧ��9���¿���ѧ�Ծ��������棩 ���ͣ�ѡ����
���й�̬CaCO3���ڵı�����Һ�У�����������ƽ��CaCO3(s) Ca2��(aq)��CO
Ca2��(aq)��CO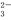 (aq)������������Һ��ʹCaCO3�������ӵ���( )
(aq)������������Һ��ʹCaCO3�������ӵ���( )
A��CaCl2��Һ B��KNO3��Һ C��NH4Cl��Һ D��NaCl��Һ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ�꽭��ʡ�߶��ϵ�һ�ζο���ѧ���������棩 ���ͣ�ѡ����
100��ʱ����0.1molN2O4����1L�ܱ������У�Ȼ����ƿ����100��ĺ��²��У���ƿ�ڵ�������Ϊ����ɫ��N2O4(g)  2NO2(g)�����н��۲���˵��������Ӧ�ڸ��������Ѿ��ﵽ��Ӧ�ȵ���
2NO2(g)�����н��۲���˵��������Ӧ�ڸ��������Ѿ��ﵽ��Ӧ�ȵ���
A��N2O4������������NO2����������֮��Ϊ1:2
B����ƿ���������ɫ���ټ���
C����ƿ�������ƽ����Է����������ٱ仯
D����ƿ�������ѹǿ���ٱ仯
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com