
| ���� | �����Լ� | ���ӷ���ʽ |
| Cu2+ | ||
| SO42- |
| n |
| V |
| n |
| V |
| n |
| V |


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��2009���ҹ�����ȼ��˰����ҵ�Ͻ�ʯ�ͷ���õ����ͣ���Ҫ�����˻�ѧ�仯 |
| B��2008��ŵ������ѧ�������о���ɫӫ�⵰�Ŀ�ѧ�ң����ֵ�������Һ����������Һ�����ö����ЧӦ |
C�� ���������������̷��¼�����Ⱦ��Ϊ�����谷���ṹ��ͼ������Ħ������Ϊl26g |
| D��2008��֧Ԯ�Ĵ�������������Ʒ��ʳ�ס����ʳ�ε���Ҫ��ѧ���ʷֱ������ᡢ��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
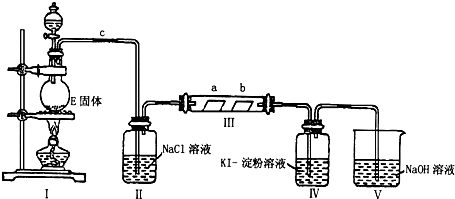
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A�������ɱ������ᡢ�ռʳ�� |
| B����ơ��������ᡢ�ռʳ�� |
| C�������������������ᡢ�������� |
| D��ͭ������ͭ�����ᡢʯ��ˮ���Ȼ�ͭ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A����ϡ�͵�����ת��������ƿ��δϴ���ձ��Ͳ����� |
| B�����ձ��ڵ�ϡ����������ƿ��ת��ʱ�����������ʹ����ϡ���ὦ��ƿ�� |
| C���ý�ͷ�ι�������ƿ�м�ˮʱ��Һ���������ƿ�̶��ߣ���ʱ�����ý�ͷ�ιܽ�ƿ�ڶ���Һ��������ʹ��Һ��Һ����̶������� |
| D���ý�ͷ�ι�������ƿ�м���ˮʱ�����ӹ۲���Һ��Һ��������ƿ�̶������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
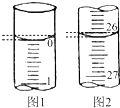 ijѧ��������֪���ʵ���Ũ�ȵ��������ⶨδ֪���ʵ���Ũ�ȵ�����������Һʱ��ѡ���̪��ָʾ��������д���пհף�
ijѧ��������֪���ʵ���Ũ�ȵ��������ⶨδ֪���ʵ���Ũ�ȵ�����������Һʱ��ѡ���̪��ָʾ��������д���пհף�| �ζ����� | ��������������Һ�����/mL | 0.1000mol/L ��������/mL | ||
| �ζ�ǰ�̶� | �ζ���̶� | ��Һ���/mL | ||
| ��һ�� | 25.00 | 0.00 | �� | �� |
| �ڶ��� | 25.00 | 1.56 | 27.68 | 26.12 |
| ������ | 25.00 | 0.22 | 26.30 | 26.08 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��Ӧ��ʹ����ȡ�ķ�������ѡ�þƾ�����ȡ�� |
| B����ȡʹ�õ���Ҫ�����Ƿ�Һ©������ʹ��ǰҪ�ȼ������Ƿ�©Һ |
| C�����÷ֲ��ⵥ��һ�������ϲ�Һ���У�Ӧ�ӷ�Һ©�����Ͽڵ��� |
| D���ӷ�Һ©���з�����ľ��Ǵ����ĵⵥ�� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com