
���к͵ζ����ⶨij�ռ���Ʒ�Ĵ���,�Ը���ʵ��ش���������:
��1��ȷ����8.2 g�������������������ʵ���Ʒ,���500 mL������Һ?����ʱ,��Ʒ�ɷ�
��________��������ĸ������?
A��С�ձ��� B���ྻֽƬ�� C��������
��2�� �ζ�ʱ,��0.2000 mol��L-1���������ζ�������Һ,����ѡ��____��������ĸ����ָʾ��?
A������ B��ʯ�� C����̪
��3��������̨�ϵ�һ�Ű�ֽ,��Ŀ����_______ _?
��4����0.20 mol��L-1���������ζ�10.00 mL������Һ���ζ���ֹʱ����������Һ20.00mL�����㱻���ռ���Һ�����ʵ���Ũ����________mol��L-1,�ռ���Ʒ�Ĵ�����________?
��5������ʵ�����������ƿ�ô���Һ��ϴ,Ȼ���ټ���10.00 mL����Һ,��ζ���� ���ƫ�ߡ�?��ƫ�͡�����Ӱ�족����
��1��A��1�֣�
��2��B ��1�֣�
��3�����ڹ۲���ƿ��Һ����ɫ�ı仯,��С�ζ���2�֣�
��4��0.40��2�֣� 97.56% ��2�֣�
��5��ƫ�ߣ�1�֣�
���������������1���ռ����ǿ��ʴ�ԣ�����ʱӦ����С�ձ��н��У�ѡA.��
��2�� ���ζ��Ĺؼ����ǵζ��յ���жϣ��ζ��յ������ָʾ����ָʾ��Ϊ�˸��õ��жϵζ��յ㣬���Dz����ܹ���dzɫ������ɫ��ָʾ��������Ҫ������ɫ����dzɫ��ָʾ��������ָʾ���ı�ɫ��ΧԽխԽ�á����ȵı�ɫ��Χ��3.1��4.4��ʯ��ı�ɫ��Χ��5��8����̪�ı�ɫ��Χ��8��10��������ζ��ռ���Һʱ��һ��ѡ����ȣ�Ҳ��ѡ���̪��������ѡ��ʯ�� ����ѡ B��
��3��������̨�ϵ�һ�Ű�ֽ��Ŀ���DZ��ڹ۲���ƿ�ڵ���ɫ�仯����С��
��4����������к͵ζ���ԭ�����ɼ��������Һ��Ũ��Ϊ��0.2mol/L��0.02L/0.01L=0.4mol/L�����ռ�����Ϊ0.4mol/L��0.5L��40g/mol=8g,������Ʒ�Ĵ���Ϊ8g/8.2g��100%=97.56%��
��5������ƿ�ô���Һ��ϴ��ʵ�����������˴���Һ�е����ʵ��������ñ�Һ���ƫ�ߣ�ʹʵ����ƫ�ߡ�
���㣺�����к͵ζ��IJ�����ָʾ����ѡ���㣬������


 ͨ��ѧ��Ĭд����ϵ�д�
ͨ��ѧ��Ĭд����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�������ƣ�NaClO2��������ˮ��������ɰ�ǡ���֬��Ư����ɱ�����������ù������ⷨ�����������ƵĹ�������ͼ��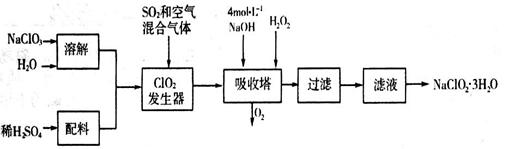
��֪��NaClO2���ܽ�����¶����߶������ʵ������¿ɽᾧ����NaClO2��3H2O��
��1���������ڷ�����Ӧ�Ļ�ѧ����ʽΪ_____________________����ƽ��ѧ����ʽ�����ù��������е�NaClO3��ClO2��NaClO2����ǿ�����������Ƕ��ܺ�Ũ���ᷴӦ��ȡCl2�����ö������Ⱥ�Ũ������ȡCl2��������5 mol Cl2ʱ��ͨ����ԭ��Ӧ�Ƶ�����������Ϊ________�ˡ�
��2������Һ�еõ���NaClO2��3H2O����IJ���������__________����д��ţ���
a.���� b.���� c.���� d.��ȴ�ᾧ
��3��ӡȾ��ҵ�����������ƣ�NaClO2��Ư��֯�Ư��֯��ʱ���������õ���HClO2���±���25��ʱHClO2�����ֳ�������ĵ���ƽ�ⳣ����
�ٳ����£����ʵ���Ũ����ȵ�NaClO2��NaF��NaCN��Na2S������Һ��pH�ɴ�С��˳��Ϊ____________�������ȣ����ʵ���Ũ����ͬ��NaF��NaCN����Һ�������������������Ĵ�С��ϵΪ��______________���ǰ�ߴ���ȡ����ߴ���
��Na2S�dz��õij�������ij��ҵ��ˮ�к��е�Ũ�ȵ�Cu2+��Fe2+��Pb2+���ӣ��μ�Na2S��Һ�����������ij�����_______�������һ�����ӳ�����ȫʱ��������Ũ��Ϊ10-5mol��L-1����ʱ��S2-��Ũ��Ϊ____��
��֪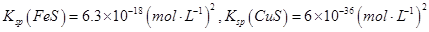
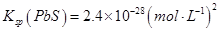
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������
�Ǒz�ᣨH3PO3)�Ƕ�Ԫ�ᣬ������NaOH��Һ��Ӧ����Na2HPO3��
��1��PCl3ˮ�����ȡ�����PCl3+3H2O=H3PO3+_______��
��2��H3PO3��Һ�д��ڵ���ƽ�⣺H3PO3 H++H2PO3����
H++H2PO3����
��ij�¶��£�0.10mol?L��1�� H3PO3��Һ pH =1.6������Һ��c(H+) =2.5��10��2 mol?L��1������¶�����������ƽ���ƽ�ⳣ��K��д��������̡���H3PO3�ĵڶ���������Բ��ƣ����������λ��Ч���֡���
�ڸ���H3PO3�����ʿ��Ʋ�Na2HPO3ϡ��Һ��pH________7 (�>������=����<������
��3�����������ǿ��ԭ�ԣ���ʹ��ˮ��ɫ���÷�Ӧ�Ļ�ѧ����ʽΪ_______��
��4�����Na2HPO3��ҺҲ�ɵõ����[�ᣬװ��ʾ��ͼ���£�
�������ĵ缫��ӦʽΪ____________________________��
�ڲ�Ʒ���з�Ӧ�����ӷ���ʽΪ_____________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
ijѧ����0.200 0 mol��L��1�ı�NaOH��Һ�ζ�δ֪Ũ�ȵ����ᣬ�����Ϊ���¼�����
��������ˮϴ�Ӽ�ʽ�ζ��ܣ�������ע��NaOH��Һ����0���̶�������
�ڹ̶��õζ��ܲ�ʹ�ζ��ܼ������Һ��
�۵���Һ������0����0���̶�������ijһ�̶ȣ������¶���
����ȡ20.00 mL����Һע��ྻ����ƿ�У�������3�η�̪��Һ
���ñ�Һ�ζ����յ㣬���µζ���Һ�����
��ش�
(1)���ϲ����д������(����) ���ô�������ᵼ�²ⶨ���(�ƫ����ƫС������Ӱ�족) ��
(2)�жϵζ��յ�������ǣ���ƿ����Һ�� ɫ��Ϊ ɫ���Ұ�����ڲ���ɫ��
(3)��ͼ��ij�εζ�ʱ�ĵζ����е�Һ�棬�����Ϊ mL��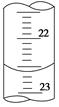
(4)�����������ݣ���������������Һ��Ũ��Ϊ mol/L
| �ζ����� | ����Һ���(mL) | ��NaOH��Һ������¼(mL) | |
| �ζ�ǰ���� | �ζ������ | ||
| ��һ�� | 20.00 | 0.40 | 20.40 |
| �ڶ��� | 20.00 | 4.00 | 24.00 |
| ������ | 20.00 | 2.00 | 24.10 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
ʵ��ⶨ����к͵ζ������Ǹ��л�ѧ����Ҫ����ʵ�顣�±�����0.10 mol/L������ζ� 0.10 mol/L 20.00 mL NaOH ��Һʱ��õ�һЩ������ݡ���ش��������⣺
��1����д���Т٢ڶ�Ӧ�� pH �����������λС����
��2����ͼ�DZ�ʵ��ĵζ�����ͼ������ݸ�ͼ��˵��ǿ����Һ�ζ�ǿ����Һʱ��Ϊʲô�ȿ���ʹ�ü�����Ϊָʾ�����ֿ���ʹ�÷�̪��Һ��ָʾ����ָʾ�ζ��յ㣿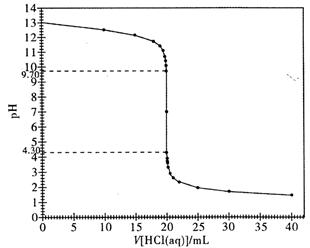
��3�����ڵζ��յ�ʱ���Ӷ��������ղ�õ�����������Һ��Ũ�Ȼ� ���ƫ����ƫС����û��Ӱ�족����ͬ�������ζ�����ʱ���ζ��ܼ������а�α����ᣬ���ղ�õ�����������Һ��Ũ�Ȼ��ʢװ����Һ����ƿϴ�Ӹɾ���δ���Tʢװ����Һ�����ղ�õ�����������Һ��Ũ�Ȼ� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
����������������Ʒ��������̨������Ȧ����ʽ���У� ����ƿ ����ʽ�ζ��ܺͼ�ʽ�ζ��� ���ձ������ɣ� �ݲ����� ��ͷ�ι� ����ƽ�������룩 ����ֽ ����Ͳ �����©����
����ҩƷ��
| A��NaOH���� | B����NaOH��Һ | C��δ֪Ũ������ | D������ˮ��E��̼������Һ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
�Ӻ�þ�����κ�ˮ���������õ��IJ����к���±ʯ(xKCl��yMgCl2��zH2O)�����ڿ����м��׳��⡢������ˮ��������طʺ���ȡ����þ����Ҫԭ�ϣ�����ɿ�ͨ������ʵ��ⶨ����ȷ��ȡ5. 550g��Ʒ����ˮ�����l00mL��Һ���ڽ���Һ�ֳɶ��ȷݣ���һ���м���������NaOH��Һ��������ȫ�����ˡ�ϴ�ӡ����������أ��õ���ɫ����0. 580g��������һ����Һ�м��������������ữ��AgNO3��Һ��������ȫ�����ˡ�ϴ�ӡ����������أ��õ���ɫ����4.305g��
��1��������м����ɫ������ϴ���ķ����� ��
��2����֪ij�¶���Mg( OH)2��Ksp ="6.4" xl0��12������Һ��c(Mg2+)��1.0��10 ��5mol��L��1����Ϊ������ȫ����Ӧ������Һ��c��OH������ mol��L��1��
��3��ͨ������ȷ����Ʒ����ɣ�д��������̣���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
��ͼ��ʾ�ĵ���I��II�У�a��b��c��d��ΪPt�缫���������У��缫b��d��û�������ݳ���������������������b>d����������ʵ����������Һ��( )
| ѡ�� | X | Y |
| A�� | AgNO3 | Cu(NO3)2 |
| B�� | MgSO4 | CuSO4 |
| C�� | FeSO4 | Al2 (SO4)3 |
| D�� | CuSO4 | AgNO3 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
��ͼ��ʾ��a��b�Ƕ��ʯī�缫��ijͬѧ��ͼʾװ�ý�������ʵ�飺 �Ͽ�K2���պ�K1һ��ʱ�䣬�۲쵽��֧�������ڶ������ݽ��缫��Χ����ʱ�Ͽ�K1���պ�K2���۲쵽������A��ָ����ƫת������˵������ȷ����
| A���Ͽ�K2���պ�K1һ��ʱ�䣬��Һ��pH��� |
| B���Ͽ�K1���պ�K2ʱ��b���ϵĵ缫��ӦʽΪ��2H++2e��====H2�� |
| C���Ͽ�K2���պ�K1ʱ��a���ϵĵ缫��ӦʽΪ��4OH����4e��====O2��+2H2O |
| D���Ͽ�K1���պ�K2ʱ��OH����b���ƶ� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com