
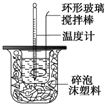
| �¶� ʵ����� | ��ʼ�¶�t1/�� | ��ֹ�� ��t2/�� | ƽ���¶Ȳ� ��t2-t1��/�� | ||
| H2SO4��Һ | NaOH��Һ | ƽ��ֵ | |||
| 1 | 26.2 | 26.0 | 26.1 | 29.5 | 3.4 |
| 2 | 27.0 | 27.4 | 27.2 | 32.3 | |
| 3 | 25.9 | 25.9 | 25.9 | 29.2 | |
| 4 | 26.4 | 26.2 | 26.3 | 29.8 | |
���� �ٲ���Ӳֽ�壬����һ��������ɢʧ��
�ڽ������ȿ죬������ʧ�ࣻ
�ۣ�i�����ж��¶Ȳ����Ч�ԣ�Ȼ������¶Ȳ�ƽ��ֵ��
��ii������Q=m•c•��T���㣬�ȸ���Q=m•c•��T���㷴Ӧ�ų���������Ȼ����ݡ�H=-$\frac{Q}{n}$kJ/mol�������Ӧ�ȣ�
��iii��a��װ�ñ��¡�����Ч�����õ�����ƫС��
b����ȡNaOH��Һ�����ʱ���Ӷ������ᵼ�������������������ƫ�ų�������ƫ�ߣ�
c���ֶ�ΰ�NaOH��Һ����ʢ�������С�ձ��У�����ɢʧ�϶ࣻ
d���¶ȼƲⶨNaOH��Һ��ʼ�¶Ⱥ�ֱ�Ӳ���ϡH2SO4���¶ȣ��������ʼ�¶�ƫ�ߣ�
��� �⣺��1���ٴ��ձ����粻��Ӳֽ�壬����һ��������ɢʧ����õ��к�����ֵ�����С��
�ʴ�Ϊ��ƫС��
�ڲ����û���ͭ˿��������滷�β������������Ϊͭ˿��������ȵ������壬������ʧ��
�ʴ�Ϊ�����ܣ�
��i��4���¶Ȳ�ֱ�Ϊ��3.4�棬6.1�棬3.3�棬3.5�棬��2������������������ɾ�����¶Ȳ�ƽ��ֵΪ$\frac{3.4��+3.3��+3.5��}{3}$=3.4�棬�ʴ�Ϊ��3.4��
��ii��0.55mol/L��NaOH��Һ50mL��0.25mol/L��������Һ50mL�����кͷ�Ӧ����ˮ�����ʵ���Ϊ0.05L��0.50mol=0.025mol����Һ������Ϊ��100ml��1g/ml=100g���¶ȱ仯��ֵ��TΪ3.4�������㣬������0.025molˮ�ų�������ΪQ=m•c•��T=100g��4.18J/��g•�棩��3.4��=1421.2J����1.421kJ������ʵ���õ��к��ȡ�H=-$\frac{1.421kJ}{0.025mol}$=-56.8 kJ/mol���ʴ�Ϊ��-56.8 kJ/mol��
��iii��a��װ�ñ��¡�����Ч�����õ�����ƫС���к��ȵ���ֵƫС����a��ȷ��
b����ȡNaOH��Һ�����ʱ���Ӷ������ᵼ�������������������ƫ�ų�������ƫ�ߣ��к��ȵ���ֵƫ��b����
c���ֶ�ΰ�NaOH ��Һ����ʢ�������С�ձ��У�����ɢʧ�϶࣬����¶�ƫ�ͣ��к��ȵ���ֵƫС����c��ȷ��
d���¶ȼƲⶨNaOH��Һ��ʼ�¶Ⱥ�ֱ�Ӳ���ϡH2SO4���¶ȣ��������ʼ�¶�ƫ�ߣ���õ�����ƫС���к��ȵ���ֵƫС����d��ȷ��
��ѡabcd��
���� ���⿼�����ʺ����IJⶨ��Ϊ��Ƶ���㣬���ط�����Ӧ�������Ŀ��飬ע�����ղⶨ�к��ȵ���ȷ��������ȷʵ����������йؼ����ھ����ܼ�������ɢʧ��ʹ�ⶨ�������ȷ��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ���÷�Ӧ����ȡ�����ȡ�����ӳɡ�����Ӧ��
���÷�Ӧ����ȡ�����ȡ�����ӳɡ�����Ӧ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | PBr3��NO2 | B�� | CH4��SCl2 | C�� | BF3��SO2 | D�� | H2O��CS2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��Ԫ�� | B�� | ��Ԫ�� | C�� | ��Ԫ�� | D�� | ��Ԫ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
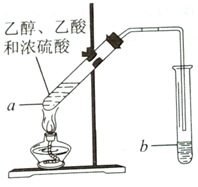 �����Ѿ��ܷⴢ��ʱ��Խ��������Խ�ã�ԭ��֮һ�Ǵ����������������ζ��������ʵ���ҿ�������ͼ��ʾ��װ����ȡ������������ش��������⣮
�����Ѿ��ܷⴢ��ʱ��Խ��������Խ�ã�ԭ��֮һ�Ǵ����������������ζ��������ʵ���ҿ�������ͼ��ʾ��װ����ȡ������������ش��������⣮�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 1.8��10-13 mol•L-1 | B�� | 7.3��10-13 mol•L-1 | ||
| C�� | 2.3 mol•L-1 | D�� | 3.7 mol•L-1 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
| �ζ� ���� | ������� ��mL�� | NaOH��Һ���������mL�� | |
| �ζ�ǰ | �ζ��� | ||
| 1 | 20.00 | 0.00 | 18.10 |
| 2 | 20.00 | 0.00 | 16.30 |
| 3 | 20.00 | 0.00 | 16.22 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
| ���� | �ܶȣ�20�棩 | �۵� | �е� | �ܽ��� |
| ������ | 0.962g/cm3 | 25.9�� | 160.8�� | 20��ʱˮ���ܽ��Ϊ3.6g���ɻ������Ҵ����� |
| ������ | 1.360g/cm3 | 152�� | 337.5�� | ��ˮ�е��ܽ�ȣ�15��ʱ1.44g��25��ʱ2.3g���������Ҵ��������ڱ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
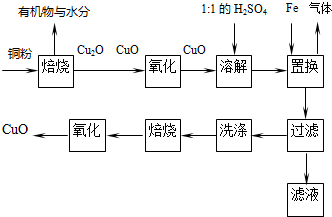
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com