
ʵ�������������������Ҵ���Ũ�������Ϊԭ���Ʊ�1,2�������顣��֪1,2���������۵�Ϊ9 �棬C2H5OH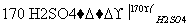 CH2===CH2����H2O��
CH2===CH2����H2O��
2C2H5OH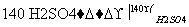 C2H5—O—C2H5��H2O
C2H5—O—C2H5��H2O
��������������������(����)
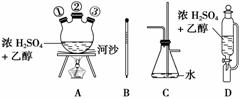

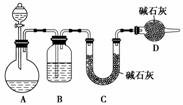
(1)�����������Ϊ�����ң���ȷ������˳����(�̽ӿڻ���Ƥ�ܾ�����ȥ)��B��A�ٲ���A�У�D��A�ڣ�A�۽�________��________��________��________��
(2)װ��A�з�Ӧ��������Ϊ________������D������Ϊ________����ɳ������Ϊ________��
(3)д������C���������ã�________________________________________________��
(4)�¶ȼ�ˮ�������ȷλ����_________________________________________________��
(5)��Ӧ��E�м�������ˮ���ѷ�Ӧ��E����ʢ����ˮ��С�ձ�������Ϊ________________________________________________________________________��
(6)��������������δ��Ӧ��Br2�������________(����ȷѡ��ǰ����ĸ)ϴ�ӳ�ȥ��
a������������������Һ
b������������Һ
c���⻯����Һ
d���Ҵ�
(7)��Ʒ�п��ܻ��е��л�������______������________������ȥ��
�𰸡�(1)C��F��E��G��
(2)������ƿ����ѹ��Һ©����������
(3)�ٰ�ȫƿ���������ڷ�ֹ����ͨ��������������Σ��
(4)Ӧ����Һ�����£����Ӵ�ƿ��
(5)���ӷ������Լ�������ӷ���ɵ���ʧ
(6)a
(7)���ѡ�����
������(1)1,2���������۵�ͣ����������ܣ�Cװ��Ӧ��������1,2��������֮ǰ����E��1,2�������������������ܣ�C��ֱ����Һ��������������ֹͣʵ�飬����ȡ��CH2===CH2�����п��ܺ���SO2���壬���ȳ�ȥ��(4)�¶ȼ��������ⶨ��ӦҺ�¶ȵģ����Ҫ����Һ�����£������ܽӴ�ƿ�ס�(5)����Һ���ӷ�������ˮҺ�⡣(6)1,2������������NaOH��Ӧ��b�����ã�c������I2����1,2���������У�d���Ҵ���1,2�������黥�ܡ�(7)����ȡ��ϩʱ���Ҵ����Ӽ���ˮ���������ѡ�


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����£���a mol N2��b mol H2�Ļ������ͨ��һ���̶��ݻ����ܱ������ڣ��������·�ӦN2+3H2 2NH3��
2NH3��
(1)����Ӧ��ijʱ��tʱ��n(N2)=13 mol�� n(NH3)=6 mol��a=_______��
(2)��Ӧ����ƽ��ʱ������������Ϊ716.8 L(��״��)����NH3�ĺ���(�������)Ϊ25%������ƽ��ʱNH3�����ʵ���_________��
(3)���������a��b=_________��
(4)ƽ����������n(N2)��n(H2)��n(NH3)=________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
A��B��C��D������ɫ��Һ�����Ƿֱ���CH3COONa��Һ��NH4Cl��Һ��NaHSO4��Һ��BaCl2��Һ�е�һ�֣���֪A��B��Һ��pH��ͬ��A��C��Ϻ���Һ����ǡ�����˵����ȷ����(����)
A��D��Һ��pH<7������������������������������B��C��Һ�е���������ˮ�ٽ���ˮ�ĵ���
C����ҺA���뵽̼��������Һ�в������塡������D����ҺB������ɫ��Ӧ�ʻ�ɫ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ij��ѧС����ʵ����������CaSO4��NH3��CO2�Ʊ�(NH4)2SO4�������������¡�
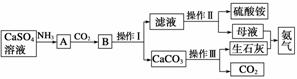
�ش��������⣺
(1)�����������________����������Ҫ�õ�������������̨(����Ȧ)���ƾ��ơ���������________��
(2)����ĸҺ�����Ե�ԭ�������ӷ���ʽ��ʾΪ______________���������Һ����笠����ӵķ�����____________________________��
(3)����װ��������ʵ�����ư�������________(�����)��
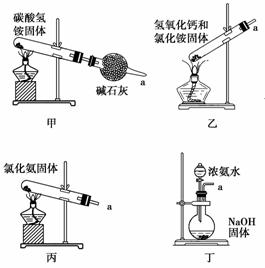
ѡ��������ȡװ�ú���ѡ������װ���ռ�����İ���������ȡ�������Һ�����ӵ�˳��(�ýӿ������ĸ��ʾ)�ǣ�a��__________________________��
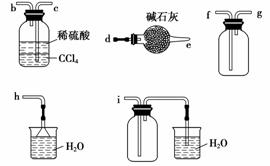
(4)��С�黹ͨ��ʵ���о����������ʡ�
������ͼ��ʾװ��̽��NH3�ܷ�NO2����(K1��K2Ϊֹˮ�У��г̶ֹ�װ����ȥ)��
Eװ������ȡNO2��Ӧ�����ӷ���ʽ��____________��A�з�����Ӧ�Ļ�ѧ����ʽΪ__________________��
��NH3�ܱ�NO2����ȫ�����������ʣ�Ԥ�ڹ۲쵽Cװ���е�������____________�����˷�Ӧת�Ƶ���0.4 mol�������ı�״���µ�NO2________ L��
ʵ������У�δ�ܹ۲쵽Cװ���е�Ԥ������С��ͬѧ�ӷ�Ӧԭ���ĽǶȷ�����ԭ����Ϊ�����ǣ�
��NO2�����Խ��������ܽ�NH3������
���ڴ������£�NH3��ת���ʼ��ͣ�
��________________________________________________________________________��
��ʵ��װ�ô���һ�����Ե�ȱ����____________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

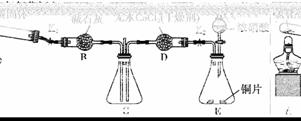
ij�о�С��Ϊ̽���������������������绯ѧ��ʴ���͵�Ӱ�����أ�����Ͼ��ȵ��������ۺ�̼��������ƿ�ײ�������ƿ��(��ͼ1)���ӽ�ͷ�ι��е��뼸�δ�����Һ��ͬʱ���������е�ѹǿ�仯��
(1)���������ʵ����Ʊ�(���в�Ҫ���ո�)��
| ��� | ʵ��Ŀ�� | ̼��/g | ����/g | ����/% |
| �� | Ϊ����ʵ�������� | 0.5 | 2.0 | 90.0 |
| �� | ����Ũ�ȵ�Ӱ�� | 0.5 | 36.0 | |
| �� | 0.2 | 2.0 | 90.0 |
(2)��Ţ�ʵ����������ѹǿ��ʱ��仯��ͼ2��t2ʱ��������ѹǿ����С����ʼѹǿ����ԭ������������________��ʴ������ͼ3���ü�ͷ��������ø�ʴʱ������������ʱ��̼�۱��淢����________(���������ԭ��)��Ӧ����缫��Ӧʽ��______________________________��

(3)��С���ͼ2��0��t1ʱѹǿ����ԭ����������¼��裬������ɼ������
����һ���������ⸯʴ���������壻
�������________________________________________________________________________��
����
(4)Ϊ��֤����һ��ijͬѧ����˼����ռ����������Ƿ���H2�ķ��������������һ��ʵ�鷽����֤����һ��д��ʵ�鲽��ͽ��ۡ�
| ʵ�鲽��ͽ���(��Ҫ��д�����������)�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ij�о���ѧϰС���ͬѧͨ���������������Ԫ�صļ�̬���з�������ΪNa2SO3��Һ�ڴ�Ź������п��ܱ��ʣ����ѱ��ʣ�����Ӧ��Ӧ�Ļ�ѧ����ʽΪ__________________________��
(1)��ͬѧ������������ַ���������Na2SO3��Һ�Ƿ���ʡ�
������ȡ��������ϡ�������������ɣ���ΪNa2SO3��Һû�б��ʡ�
������ȡ���������Ȼ�����Һ�а�ɫ�������ɣ���ΪNa2SO3��Һ��ȫ���ʡ�
��ͬѧ��Ϊ�������ַ���������������ͬѧ�����жϵ����ݷֱ��Ƿ�����________��������________��
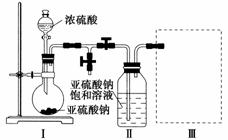
(2)��ͬѧ�����ͼ��ʾװ��(�г�װ������ȥ)̽��Na2SO3��Һ�Ƿ���ʣ���ȡ60 mL��ǩ��ע��Ϊ1.0 mol·L��1��Na2SO3��Һ����Aװ���У�ͨ���������ɵ�SO2�����ķ������ж����Ƿ���ʡ�
��Dװ�õ�������________����ʵ��װ���л�����һ�����Ե�ȱ����________________����Һ©����Ӧ����������________(����ĸ)��
a��Ũ���� b��65%����
c��Ũ���� d��20%����
�ڸĽ�ȱ�ݺ��ٽ���ʵ�飬��ʵ��ǰ����Cװ������3.2 g����Na2SO3���ʵİٷ�����__________��
(3)��ͬѧ��Ϊ��ֻ��ȷ��Na2SO3�Ƿ���ʣ��ҵķ���̫�����ˣ�����Ϊֻ��Ҫ�Թܡ���ͷ�ιܡ����ἰ�Ȼ�����Һ�Ϳ��ԴﵽĿ��(�����ǶԻ�����Ӱ��)�����㷢���Լ��Ŀ���(����ͬ�⣬��˵�����ɣ���ͬ�⣬��д����Ҫ������������)��______________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
һ�������·�ӦmA(g)+nB(g)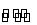 pC(g)+qD(g)��һ�ܱ������н��У����ƽ����Ӧ����v(C)=2v(B)������Ӧ��ƽ����¶Ȳ��䣬�Ӵ���ϵѹǿʱƽ�ⲻ�ƶ�����m��n��p��q����ֵ������( )
pC(g)+qD(g)��һ�ܱ������н��У����ƽ����Ӧ����v(C)=2v(B)������Ӧ��ƽ����¶Ȳ��䣬�Ӵ���ϵѹǿʱƽ�ⲻ�ƶ�����m��n��p��q����ֵ������( )
A��2��6��3��5 B��3��1��2��1
C�� 3��1��2��2 D��1��3��2��2
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����������(Na2S2O5)�dz��õ�ʳƷ��������֮һ��ij�о�С���������ʵ�飺
ʵ��һ�����������Ƶ���ȡ
������ͼװ��(ʵ��ǰ�ѳ���װ���ڵĿ���)��ȡNa2S2O5��װ�â�����Na2S2O5���������������ķ�ӦΪNa2SO3��SO2===Na2S2O5��
(1)װ�â��в�������Ļ�ѧ����ʽΪ________________________��
(2)Ҫ��װ�â��л���������ľ��壬�ɲ�ȡ�ķ��뷽����________________________��
(3)װ�â����ڴ���β������ѡ�õ������װ��(�г���������ȥ)Ϊ________(�����)��
ʵ��������������Ƶ�����
Na2S2O5����ˮ������NaHSO3��
(4)֤��NaHSO3��Һ��HSO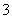 �ĵ���̶ȴ���ˮ��̶ȣ��ɲ��õ�ʵ�鷽���� ________ (�����)��
�ĵ���̶ȴ���ˮ��̶ȣ��ɲ��õ�ʵ�鷽���� ________ (�����)��
a���ⶨ��Һ��pH
b������Ba(OH)2��Һ
c����������
d������Ʒ����Һ
e������ɫʯ����ֽ���
(5)����Na2S2O5�����ڿ������ѱ�������ʵ�鷽����________________________��
ʵ���������Ѿ��п��������������IJⶨ
(6)���ѾƳ���Na2S2O5�������������ⶨij���Ѿ��п��������IJ�����(������SO2����)�ķ������£�
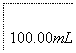
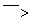

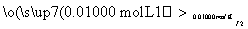 ��Һ������ɫ��30 s�ڲ���ɫ
��Һ������ɫ��30 s�ڲ���ɫ
(��֪���ζ�ʱ��Ӧ�Ļ�ѧ����ʽΪSO2��I2��2H2O===H2SO4��2HI)
�ٰ���������ʵ�飬���ı�I2��Һ25.00 mL���ô�ʵ������Ʒ�п��������IJ�����(������SO2����)Ϊ________g��L��1��
��������ʵ������У����в���HI��������������ⶨ���________(�ƫ�ߡ���ƫ�͡����䡱)��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com