
��ú��Ϊȼ�Ͽ�ͨ����������;����
;��I��C(s) +O2 (g)=CO2(g) ��H1<0 ��
;��II�����Ƴ�ˮú����C(s) +H2O(g)=CO(g)+H2(g) ��H2>0 ��
��ȼ��ˮú����2CO(g)+O2 (g)=2CO2(g) ��H3<0 ��
2H2(g)+O2 (g)=2H2O(g) ��H4<0 ��
��ش��������⣺
��1��;��I�ų������� ( ����ڡ������ڡ���С�ڡ�) ;��II�ų���������ԭ���� ��
��2����H1����H2����H3����H4����ѧ��ϵʽ�� ��
��3��12g̿���������в���ȫȼ������һ����̼���ų�110.35kJ���������Ȼ�ѧ����ʽΪ ��
��4��ú̿��Ϊȼ�ϲ���;��II���ŵ���
��1�����ڣ�2�֣������ݸ�˹���ɿ�֪�����һ����ѧ��Ӧ���Էֲ����У�����ֲ���Ӧ�ķ�Ӧ��֮����÷�Ӧһ�����ʱ�ķ�Ӧ������ͬ�ġ���3�֣���˼�Լ���
��2����H1=��H2+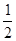 ����H3+��H4�� ��4�֣�
����H3+��H4�� ��4�֣�
��3��C(s) +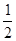 O2 (g)= CO(g) ��H="-110.35" kJ��mol-1 ��4�֣�
O2 (g)= CO(g) ��H="-110.35" kJ��mol-1 ��4�֣�
��4��ȼ��ȼ�ճ�֣������ʸߣ����ȶ࣬��ȾС����3�֣�
���������������1����2�����ݸ�˹���ɿ�֪�����һ����ѧ��Ӧ���Էֲ����У�����ֲ���Ӧ�ķ�Ӧ��֮����÷�Ӧһ�����ʱ�ķ�Ӧ������ͬ�ġ��ʴ��ڡ�H1=��H2+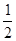 ����H3+��H4����;��I�ų�����������;��II�ų���������
����H3+��H4����;��I�ų�����������;��II�ų���������
��3��n��C��=1mol��C(s) +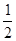 O2 (g)= CO(g) ��H="-110.35" kJ��mol-1
O2 (g)= CO(g) ��H="-110.35" kJ��mol-1
��4��ȼ��ȼ�ճ�֣������ʸߣ����ȶ࣬��ȾС��
���㣺��ѧ��Ӧ����ЧӦ



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
2013��12��2�գ��ҹ����������Ƿ��������á��������żס����ػ�������϶����š�̽�����dzɹ�����̫�գ���һ����㺮��̽�������������żס�������Һ�����ƻ����һ������Ϊ����ȼ�ϣ�����ȼ��ͨ��ָ���£�N2H4��Ϊȼ�ϣ��Զ�����������������
����ȼ��ͨ��ָ���£�N2H4��Ϊȼ�ϣ��Զ�������������������������Ϊ���÷��������������������������Ӧ�ͷŵ������������߷�Ӧ���ɵ����ͷ��������壩��
��֪��N2H4(g)+O2(g)=N2(g)+2H2O(g) ��H = ��543kJ��mol��1 H2(g)+
H2(g)+  F2(g) = HF(g) ��H = ��269kJ��mol��1
F2(g) = HF(g) ��H = ��269kJ��mol��1
H2(g)+  O2(g) = H2O(g) ��H = ��242kJ��mol��1
O2(g) = H2O(g) ��H = ��242kJ��mol��1
��д���ºͷ�����Ӧ���Ȼ�ѧ����ʽ��_____________________________��
��������������������NO�� O2���ɣ���֪��2 L�ܱ������ڣ�800 ��ʱ��Ӧ��
2NO(g)��O2(g) 2NO2(g) ��H ����ϵ�У�n(NO)��ʱ��ı仯�����
2NO2(g) ��H ����ϵ�У�n(NO)��ʱ��ı仯�����
| ʱ��(s) | 0 | 1 | 2 | 3 | 4 | 5 |
| n(NO)(mol) | 0.200 | 0.100 | 0.080 | 0.050 | 0.050 | 0.050 |
| n(O2)(mol) | 0.100 | 0.050 | 0.040 | 0.025 | 0.025 | 0.025 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
SO2��NOx�ڻ�ѧ��ҵ������Ҫ��;��Ҳ�Ǵ�����Ⱦ����Ҫ��Դ�����������ò��أ�Ԥ�������������ǵ�ǰ��ҵ�Ϻͻ������������о�����Ҫ����֮һ��
��1���ڽӴ���������Ĺ����У�����2SO2(g)��O2(g)  2SO3(g) ��H��0��Ӧ��ij�¶��£�SO2��ƽ��ת���ʣ���������ϵ��ѹǿ��p���Ĺ�ϵ����ͼ��ʾ������ͼʾ�ش��������⣺
2SO3(g) ��H��0��Ӧ��ij�¶��£�SO2��ƽ��ת���ʣ���������ϵ��ѹǿ��p���Ĺ�ϵ����ͼ��ʾ������ͼʾ�ش��������⣺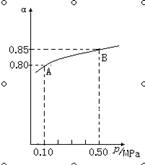
��ƽ��״̬��A��Bʱ��ƽ�ⳣ��K(A) K(B)�����������������������
�ڽ�2��0molSO2��1��0molO2����10L���ܱ������У���40s��Ӧ�ﵽƽ�⣬��ʱ��ϵ��ѹǿΪ0��10MPa����һ��ʱ����SO2��ƽ����Ӧ����Ϊ ��
�÷�Ӧ��ƽ�ⳣ��Ϊ ��
��2����CH4����ԭNOx�������������������Ⱦ�����磺CH4(g)��4NO2(g) �� 4NO(g)��CO2(g)��2H2O(g) ��H��-574kJ��mol��1
CH4(g)��4NO(g) �� 2N2(g)��CO2(g)��2H2O(g) ��H��-1160kJ��mol��1
ȡ��״����4��48LCH4��ʹ֮��ȫ��Ӧ��
������NO2��ԭ��N2������������ת�Ƶ��ӵ����ʵ���Ϊ ��
������ԭNO2��NO�Ļ����ų���������Q��ȡֵ��Χ�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��1����֪���������Ȼ�ѧ����ʽ��
C3H8(g)+5O2(g) =3CO2(g)+4H2O(l) ��H=��2220.0 kJ��mol-1
H2O��l��=H2O��g�� ��H="+44.0" kJ��mol-1
��0.5 mol����ȼ������CO2����̬ˮʱ�ͷŵ�����Ϊ___________ ��
��2����ѧ���ѻ���˼��������о������N4���ӣ���ṹΪ�������壨����ͼ��ʾ��������������ơ���֪����1molN��N������193kJ����������1molN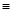 N������941kJ��������1molN4����ת��Ϊ2molN2ʱҪ�ų�______________ kJ������
N������941kJ��������1molN4����ת��Ϊ2molN2ʱҪ�ų�______________ kJ������
��3������������ɴ���ʹ�õ�������ȼ�ϵ�أ������ܷ�ӦΪ��
2H2+O2=2H2O���������ҺΪϡH2SO4��Һ����طŵ�ʱ�ǽ�_________��ת��Ϊ___________�ܡ���缫��Ӧʽ�ֱ�Ϊ��
����_________________________������_____________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�����仯����������������������е���ϵ��������һ֧15mL���Թܣ�����NO������ˮ���У����Թ��л���ͨ��һ�������������Թ���Һ���ȶ�ʱ��ʣ������3mL����ͨ���������������Ϊ mL��
��Ŀǰ����������������Ⱦ�ж��ַ�����
��1����CH4����ԭ��������������������������Ⱦ����֪��
��CH4(g)+4NO2(g)= 4NO(g)+CO2(g)+2H2O(g) ��H="-574kJ/mol"
��CH4(g)+4NO(g)= 2N2(g)+CO2(g)+2H2O(g) ��H="-1160kJ/mol"
��H2O(g)= H2O(l) ��H=-44kJ/mol
д��CH4(g)��NO2(g)��Ӧ����N2(g) ��CO2(g)��H2O(l)���Ȼ�ѧ����ʽ ��
��2���û���̿��ԭ��������������йط�ӦΪ��
ij�о�С��������ܱ���������һ�����Ļ���̿��NO�����£�T0C)�����·�Ӧ����Ӧ���е���ͬʱ���ø����ʵ�Ũ�����£�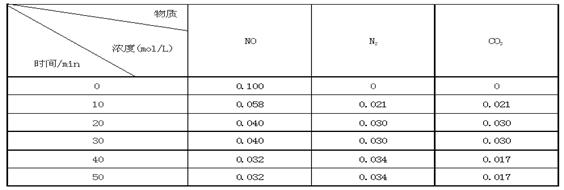
�ٲ�����Ϊ�жϷ�Ӧ�ﵽ��ѧƽ��״̬��������_______ ����ѡ����ĸ���ţ�
A��������CO2��Ũ�ȱ��ֲ���
B��v��(N2)="2" v��(NO)
C��������ѹǿ���ֲ���
D�����������ܶȱ��ֲ���
E����������ƽ����Է����������ֲ���
��ǰ20���ӣ�ƽ����Ӧ����v(NO)= ��v(NO)=��0��1- 0��04��/ 20 = 0��003mol��L-1�� min-1
����T0Cʱ���÷�Ӧ��ƽ�ⳣ��Ϊ_______(������λС��)��
����30 min���ı�ijһ������Ӧ���´ﵽƽ�⣬��ı��������_______ ��
��3����ѧ�������о����ô������������ٷɻ�β���е�NO��COת���CO2��N2,�䷴ӦΪ�� 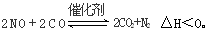
�о���������ʹ�õ���������ʱ����������ıȱ���������ѧ��Ӧ���ʡ�Ϊ�˷ֱ���֤�¶ȡ������ıȱ�����Ի�ѧ��Ӧ���ʵ�Ӱ����ɣ�ijͬѧ���������ʵ�飬����ʵ�������Ѿ������±��С�
| ʵ���� | T(0C) | NO��ʼŨ�� ��mol/L�� | CO��ʼŨ�� ��mol/L�� | �����ı� �����(m2/g) |
| �� | 280 | 1��20��10-3 | 5��80��10-3 | 82 |
| �� | a | b | c | 124 |
| �� | 350 | d | e | 124 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
Ħ��������˾������һ���Լ״�Ϊԭ�ϣ���KOHΪ����ʵ������ֻ��Ŀɳ��ĸ�Чȼ�ϵ�أ���һ�ε��������ʹ��һ���¡���֪�õ�ص��ܷ�ӦʽΪ��
2CH3OH��3O2��4KOH 2K2CO3��6H2O
2K2CO3��6H2O
����գ�
(1)�ŵ�ʱ�������ĵ缫��ӦʽΪ_________________________________________________________��
(2)ͨ��״�һ�˵ĵ缫��________��������ڷŵ��������Һ��pH��_______ (������������½������䡱)��
(3)���ڳ��¡���ѹ�£�1 g CH3OHȼ������CO2��Һ̬ˮʱ�ų�22.68 kJ����������ʾ�÷�Ӧ���Ȼ�ѧ����ʽΪ________________________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�״���һ������ȼ�ϣ�������ȼ�ϵ�ء�
��1��Ϊ̽����CO2����ȼ�ϼ״��ķ�Ӧԭ�����ֽ�������ʵ�飺�����Ϊ1L���ܱ������У�����1molCO2��3molH2��һ�������·�����Ӧ��CO2(g)��3H2(g) CH3OH(g)��H2O(g) ��H����49.0kJ/mol
CH3OH(g)��H2O(g) ��H����49.0kJ/mol
���CO2��CH3OH(g)��Ũ����ʱ��仯����ͼ����ش�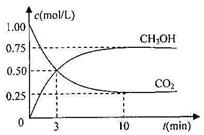
�ٴӷ�Ӧ��ʼ��ƽ�⣬�����ķ�Ӧ���ʣ�v(H2)�� ��
���ܹ�˵���÷�Ӧ�Ѵﵽƽ�����_________��
A�����¡�����ʱ�������ڵ�ѹǿ���ٱ仯
B�����¡�����ʱ�������ڻ��������ܶȲ��ٱ仯
C��һ�������£�CO��H2��CH3OH��Ũ�ȱ��ֲ���
D��һ�������£���λʱ��������3molH2��ͬʱ����1molCH3OH
�����д�ʩ����ʹƽ��������n(CH3OH)/n(CO2)������� ��
A��������� B������He(g)��ʹ��ϵѹǿ����
C����H2O(g)����ϵ�з��� D�������¶�
������¶�(T1)�¸÷�Ӧ��ƽ�ⳣ��K1�� (������������λ��Ч����)��
�������¶�(T2)�����²��ƽ�ⳣ��K2����֪T2��T1����K2 (�����������������)K1��
��2����CH3OHΪȼ��(��KOH��Һ���������Һ)���Ƴ�CH3OHȼ�ϵ��(����ܷ�Ӧʽ��2CH3OH��3O2��4OH����2CO32����6H2O)�������CH3OH�ĵ缫Ϊ ��������O2�ĵ缫��Ӧʽ ��
��3����֪�ڳ��³�ѹ�£�
��2CH3OH(l)��3O2(g)��2CO2(g)��4H2O(g) ��H1
��2CO(g)+O2(g)��2CO2(g) ��H2
��1mol�״�����ȫȼ������һ����̼����̬ˮʱ��Ӧ�ġ�H�� �����ú���H1����H2��ʽ�ӱ�ʾ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��1����֪���ױȰ����ȶ�����֪��4P(���ף�s��+5O2��g����2P2O5��s�� ��H1��
4P�����ף�s��+5O2��g����2P2O5��s�� ��H2����H1�ͦ�H2�Ĺ�ϵ�ǡ�H1 ��H2���������������
��������
��2����֪H2��g����CH3OH��l����ȼ���ȡ�H�ֱ�Ϊ-285.8kJ��mol-1��-726.5kJ��mol-1��д����CO2
��H2����Һ̬�״���Һ̬ˮ���Ȼ�ѧ����ʽ ��
��3����֪һ���¶��£����з�Ӧ��ƽ�ⳣ����SO2(g)+1/2O2(g)  SO3(g) K1,CO(g)+1/2O2(g)
SO3(g) K1,CO(g)+1/2O2(g)  CO2(g) K2������ͬ�¶��·�ӦSO2(g)+CO2(g)
CO2(g) K2������ͬ�¶��·�ӦSO2(g)+CO2(g)  SO3(g)+CO(g)��ƽ�ⳣ��Ϊ ��
SO3(g)+CO(g)��ƽ�ⳣ��Ϊ ��
����K1��K2��ʾ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��1����֪2H2��g��+O2��g��=2H2O��l����H����571.6 kJ/mol��
CO��g����1/2O2��g����CO2��g����H����283.0 kJ/mol��ijH2��CO�Ļ��������ȫȼ��ʱ�ų�113.74 kJ������ͬʱ����3.6 gҺ̬ˮ����ԭ���������H2��CO�����ʵ���֮��Ϊ___________��
��2���Լ״�������������������ҺΪԭ�ϣ�ʯīΪ�缫���ɹ���ȼ�ϵ�أ���֪��ȼ�ϵ�ص��ܷ�Ӧʽ�ǣ�2CH3OH +3O2+4OH-=2CO32-+6H2O����ȼ�ϵ�ط�����Ӧʱ����������Һ��PH__________ (����� ����С�� ���䡱)�õ�صĸ�����ӦʽΪ_________________��
��3�� ������ȼ�ϵ�ؽ��д�ͭ�ľ�������ͭӦ���ӵ�Դ��________�����ô�ͭ�������ص�������ӦʽΪ_________________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com