
��֪��C2H5OH+HBr��C2H5Br+H2O��Ϊ֤���Ҵ������к�����ԭ�ӣ��ֲ���һ��װ�ý���ʵ�顣�Ը�������װ��ͼ���Լ���ʵ�����ش��й����⡣
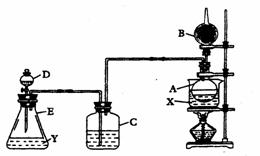 ��.װ������װ���Լ��ǣ���Aƿװ��ˮ�Ҵ����ڷ���ˮ��X����B�������װ��ʯ�ң�
��.װ������װ���Լ��ǣ���Aƿװ��ˮ�Ҵ����ڷ���ˮ��X����B�������װ��ʯ�ң�
��C��D�ж�װŨ�����Eƿ��װ���Լ�Y
ʵ������������ǣ�
��.��ˮԡ����Aƿ����D��Ũ���Ỻ������E�����Լ�Y���ã�����C�е����д������ݷų���Aƿ��X��ɫ����B�ܿڻӷ�������ɵ�ȼ����ش����и����⣺
��1��Eƿ����װ���Լ�Y�����µ�???????________
A.����ʳ��ˮ�������� �� B.MnO2��NaCl�Ļ����������� C.Ũ����
��2��Dƿ��Ũ���������������_____�������� __ ;Cƿ��Ũ���������������________ ___�������������� ___
��3��Aƿ�з�����Ӧ�Ļ�ѧ����ʽ��________________________________����Ӧ������____________�������ɵ�__________��д���ƣ���B���ڴ���ȼ��
��4����ˮ��X��ѡ��_________������ָʾ���õ�ԭ����__���������������� ______
��5����ʵ����֤���Ҵ������к�����ԭ�ӵ�������__________________________________
(6)�����װ���е�Cƿȡ����ʵ��Ŀ���Ƿ��ܴﵽ________����Ϊ_____������ ___������������������������������ ����
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ���ȶ����Զ�ʧˮ |



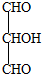
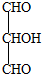




�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
 ��1����˹������Ϊ�����ܻ�ѧ������һ����ɻ��������ɣ��������̵���
��1����˹������Ϊ�����ܻ�ѧ������һ����ɻ��������ɣ��������̵����鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(1)д������ɫֲ��Ľո�ת��Ϊ�Ҵ��Ļ�ѧ����ʽ��________________________________��
(2)��֪��C2H5OH(l)+3O2(g)![]() 2CO2(g)+3H2O(l)����H=-1 367 kJ��mol-1
2CO2(g)+3H2O(l)����H=-1 367 kJ��mol-1
CH4(g)+2O2(g)![]() CO2(g)+2H2O(l)����H=-890 kJ��mol-1
CO2(g)+2H2O(l)����H=-890 kJ��mol-1
��ij��ֲ��ĽոѺ���ά��Լ50%����ֲ��ոѾ���һϵ��ת���õ��Ҵ�ԭ�ϵ���������Ϊ80%������1 000 g�ո�Ϊԭ���Ƶõ��Ҵ�ȼ��ȼ����������������________L(��״����)������ȫȼ�ղ����������൱��
(3)��ɫֲ�������õ�Ч����________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013��ɽ��ʡ����ʵ����ѧ������һ��ģ�⿼�Ի�ѧ�Ծ����������� ���ͣ���ѡ��
��֪��C2H5OH(g)+3O2(g) ="==" 2CO2(g)+3H2O(g) ��H1��-Q1kJ��mol-1��C2H5OH(g) ="==" C2H5OH(l) ��H2��-Q2kJ��mol-1��H2O(g) ="==" H2O(l) ��H3��-Q3kJ��mol-1����ʹ23gҺ��ƾ���ȫȼ�գ��ָ������£���ų�������Ϊ �� ��
| A��Q1��Q2��Q3 |
| B��0.5Q1��0.5Q2��1.5Q3 |
| C��0.5��Q1��Q2��Q3�� |
| D��0.5Q1��1.5Q2��0.5Q3 |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com