
 =______________��
=______________�� 


| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
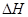 �ľ���ֵ����ȷ��
�ľ���ֵ����ȷ��| A��C2H5OH��l��+3O2��g��==2CO2��g��+3H2O��g������H=��1367.0 kJ/mol��ȼ���ȣ� |
| B��NaOH��aq��+HCl��aq��==NaCl��aq��+H2O��l������H=+57.3kJ/mol���к��ȣ� |
| C��S��s��+O2��g��===SO2��g������H=��269.8kJ/mol����Ӧ�ȣ� |
| D��2NO2==O2+2NO����H=+116.2kJ/mol����Ӧ�ȣ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
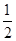 O2(g)===H2O(l)����H3����285.8 kJ/mol
O2(g)===H2O(l)����H3����285.8 kJ/mol| A��488.3 kJ/mol | B����224.15 kJ/mol |
| C����488.3 kJ/mol | D��244.15 kJ/mol |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
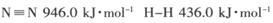
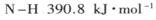 ��
�� 

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��������ȼ����Ϊ-285.5kJ��mo1����ˮ�����Ȼ�ѧ����ʽΪ�� 2H2O��1�� =2H2��g��+O2��g������H=+285.5KJ��mo1 |
| B��1mol������ȫȼ������CO2��H2O��1��ʱ�ų�890kJ�����������Ȼ�ѧ����ʽΪ 1/2CH4��g��+O2��g��= 1/2CO2��g��+H2O��1������H= һ445kJ��mol |
| C����֪2C��s��+O2��g��=2CO��g������H= һ221kJ��mol-1����C��ȼ����Ϊһ110.5kJ��mo1 |
| D��HF��NaOH��Һ��Ӧ��H+��aq��+OH����aq��=H2O��1������H= һ57.3kJ��mol |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
 2NH3�����ֱ���t��ʱ�ⶨ����NH3�������������ͼ���ң�
2NH3�����ֱ���t��ʱ�ⶨ����NH3�������������ͼ���ң�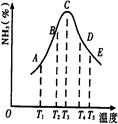
| A�������¶� | B�������¶� | C������ѹǿ | D����Сѹǿ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��-488.3 kJ��mol��1 | B��-244.15 kJ��mol��1 | C��+488.3 kJ��mol��1 | D��+244.15 kJ��mol��1 |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com