
��.��4�֣�ʵ������һƿ�������Һ��ʵ����Աȷ�����п��ܺ���NH4+��K+��Na+��Mg2+��Ba2+��Al3+��Fe3+��Cl-��I-��NO3-��CO32-��SO42-��ȡ����Һ��������ʵ�飺
��ȡpH��ֽ���飬������Һ��ǿ���ԡ�
��ȡ��������Һ����������CCl4������������ˮ������CCl4����Ϻ�ɫ��
����ȡ������Һ����NaOH��Һ��ʹ��Һ��������Ϊ���ԣ��ڵμӹ����к͵μ���Ϻ���Һ������������
��ȡ��������������Һ��Na2CO3��Һ���а�ɫ�������ɡ�
�ݽ��۵õ��ļ�����Һ���ȣ�������ų�����������ʹʪ��ĺ�ɫʯ����ֽ������
����ڵ�ˮ���м���HNO3�ữ��AgNO3��Һ�а�ɫ������
��������ʵ����ʵȷ���������жϸ���Һ��
��1���϶����ڵ������� ��
��2������ȷ���Ƿ���ڵ������� ��
��. ��6�֣���������(SeO2)��һ�����������䱻��ԭ��ĵ��������ܳ�Ϊ������Ⱦ�ͨ����ŨHNO3��ŨH2SO4��Ӧ����SeO2�Ի���Se�����������գ�
��1��Se��ŨHNO3��Ӧ�Ļ�ԭ����ΪNO��NO2����NO��NO2�����ʵ���֮��Ϊ1��1��д��Se��ŨHNO3�ķ�Ӧ����ʽ ��
��2����֪��Se+2H2SO4(Ũ)��2SO2��+SeO2+2H2O��2SO2+SeO2+2H2O��Se+2SO42-+4H+
SeO2��H2SO4(Ũ)��SO2����������ǿ������˳���� ��
��3�����յõ���SeO2�ĺ���������ͨ������ķ����ⶨ��
��SeO2+ 4KI+ 4HNO3��Se+2I2+ 4KNO3+2H2O ��I2+2Na2S2O3��Na2S4O6+2NaI
ʵ���У�ȷ����SeO2��Ʒ0.1500g��������0.2000 mol/L��Na2S2O3��Һ25.00 mL�����ⶨ����Ʒ��SeO2����������Ϊ ��
��.��4�֣���1��I-��Ba2+��NH4+(2��)��2��K+��Na+��Cl?(2��)
��. ��6�֣���1��Se��2HNO3(Ũ)=SeO2��NO����NO2����H2O (2��)
��2��H2SO4(Ũ)��SeO2��SO2(2��)��3��92.5%(2��)
���������������.����ʵ�飨1��������Һ��ǿ���ԣ�˵����Һ�п϶�����H+����H+��CO32-������Ӧ�����ܹ��棬˵����Һ�п϶�������CO32-������ʵ�飨2������CCl4����Ϻ�ɫ��˵����I2����������I-�����������������ģ��Ӷ�˵����Һ�к���I-����I-��Fe3+��NO3-��H+-������Ӧ�����ܹ��棬˵����Һ�п϶�������Fe3+��NO3-������ʵ�飨3��������Һ��������Ϊ���ԣ��ڵμӹ����к͵μ���Ϻ���Һ��������������Mg2+��Al3+����Ӧ����������˵����Һ�п϶�������Mg2+��Al3+������ʵ�飨4������ȡ����������������Һ��Na2CO3��Һ���а�ɫ�������ɣ�˵����Һ�п϶�����Ba2+����Ba2+����SO42-����������˵����Һ�в���SO42-������ʵ�飨5����������������ʹʪ��ĺ�ɫʯ����ֽ�������������Ϊ������˵����Һ�п϶���NH4+������ʵ�飨6��������ڼ�������ˮ��������Cl?��������ԭ��Һ�Ƿ���Cl?��������������1�����ṩ�������п϶����е�����Ϊ��I-��NH4+��Ba2+����2������ȷ��������Ϊ��K+��Na+��Cl-��
��. ��1������������Ϣ��֪Se��ŨHNO3��Ӧ��Se������Ϊ+4�۵�H2SeO3��HNO3��ԭΪNO��NO2������NO��NO2�����ʵ���֮��Ϊ1��1����������ϵ����Ϊ1��1�������ϵ��Ϊ1�����ݵ���ת���غ��֪��Se��ϵ��Ϊ��1��3+1��1����4=1���ʷ�Ӧ����ʽΪ��Se+2HNO3��Ũ��=H2SeO3+NO��+NO2����
��2����������ԭ��Ӧ�У���������������ǿ����������������ԣ����Ը��ݷ�Ӧ�ķ���ʽ��֪��SeO2��H2SO4��Ũ����SO2����������ǿ������˳����H2SO4��Ũ����SeO2��SO2��
��3�����ݷ�Ӧ�ķ���ʽ��֪SeO2��2I2��4Na2S2O3�����ĵ�n��Na2S2O3��=0.2000 mol/L��0.025L=0.005mol�����ݹ�ϵʽ������Ʒ��n��SeO2��=0.005mol��1/4=0.00125mol����SeO2������Ϊ0.00125mol��111g/mol=0.13875g��������Ʒ��SeO2����������Ϊ0.13875g��0.1500g��100%=92.5%��
���㣺���⿼�������ƶϡ���ѧ����ʽ����д�������Ե�ǿ���жϡ����ݹ�ϵʽ�ļ��㡣


 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
ij��Һ���ܺ���Cl����SO42����CO32����NH4+��Fe3+��Fe2+��Al3+��Na+��ijͬѧΪ��ȷ����ɷ֣�ȡ������Һ����Ʋ����������ʵ�飺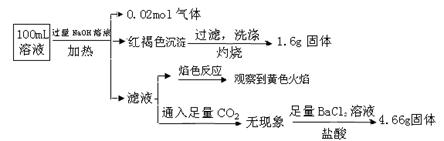
�ɴ˿�֪ԭ��Һ��
| A��ԭ��Һ��c��Fe3+��="0.2" mol��L-1 |
| B����Һ��������4�����Ӵ��ڣ�����Cl��һ�����ڣ���c��Cl������0.2 mol��L-1 |
| C��SO42����NH4+��Na+һ�����ڣ�CO32����Al3+һ�������� |
| D��Ҫȷ��ԭ��Һ���Ƿ���Fe2+,�����Ϊ��ȡ����ԭ��Һ���Թ���,����������ˮ���������ټ�KSCN��Һ����Һ��Ѫ��ɫ������Fe2+ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
ij��ɫ����Һ�п��ܺ���Ag+��Fe3+��K+��Ba2+��NH4+�������ӡ�ijͬѧ��������ʵ�飺
I�����������ϡ���ᣬ�а�ɫ�������ɡ�
II�����ˣ�ȡ������Һ�������м��������ϡ���ᣬ���а�ɫ�������ɡ�
III����ȡ��������II�е���Һ������NaOH��Һ����Һ�ʼ��ԣ����ȣ��ɲ���ʹʪ��ĺ�ɫʯ����ֽ����ɫ�����塣
��1������Һ��һ�����е�������__________��һ�������е�������___________��
��2������III�в�����������ӷ���ʽΪ__________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
����Ũ�ȷֱ�Ϊ1 mol/L��FeCl3��FeCl2��CuCl2�����Һ100 mL������һ���������ۣ������������ա�
��1����ַ�Ӧ�������Һ�л���һ������Cu2��������Һ��һ�����еĽ������ӻ���___________������������Һ�е����ʵ�����ΧΪ________________�����ܺ��еĽ������������Ϊ____________��
��2����Ӧ��Ϻ�������ʣ�࣬��Һ��һ�����еĽ�������Ϊ___________��Ϊ______mol��
һ��û�еĽ�������Ϊ______________��
��3������FeCl3��Һ�м�����������ᣬ������Ӧ�����ӷ���ʽΪ____________________��
��4��ijһ��Ӧ��ϵ���з�Ӧ��������ﹲ�������ʣ�S��H2S��HNO3��NO��H2O���÷�Ӧ�Ļ�ѧ����ʽΪ________________________________������Ӧ������ת����0.3mol���ӣ������������������___________g�����ɵ������ڱ���µ������________L��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��һ��ɫ������Һ��Ҫȷ���Ƿ����������ӣ�K����Mg2����Al3����Fe2����Ba2����NO3����SO42����Cl����I����HCO3����ȡ����Һʵ�����£�
| ʵ�鲽�� | ʵ������ |
| ��1��ȡ��������Һ���Ӽ��μ��� | ��Һ���ɫ |
| ��2��ȡ��������Һ����Ũ������CuƬ��ŨH2SO4������ | ����ɫ����������������������Ա�ɺ���ɫ |
| ��3��ȡ��������Һ����BaCl2��Һ | �а�ɫ�������� |
| ��4��ȡ��3�����ϲ���Һ����AgNO3��Һ | ���ȶ��İ�ɫ�������ɣ��Ҳ�����HNO3 |
| ��5��ȡ��������Һ����NaOH��Һ | �а�ɫ�������ɣ���NaOH����ʱ���������ܽ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
ij�о�С���û�����(FeS2)�������ƺ�������Һ��Ϸ�Ӧ�Ʊ�ClO2���壬����ˮ���ո�����ɵ�ClO2��Һ���ڴ˹�������Ҫ�������˵��¶ȣ����¶Ȳ���������Ӧ���ӣ�Ӱ������ClO2����Ĵ��ȣ��һ�Ӱ��ClO2�������ʣ����������ͼ��ʾ��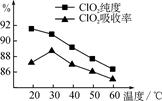
��1�� ��ͼ��֪����Ӧʱ��Ҫ���Ƶ������¶���________�棬Ҫ�ﵽ��Ҫ����Ҫ��ȡ�Ĵ�ʩ��______________��
��2�� ��֪���������е���Ԫ�������������¿ɱ�ClO3-������SO42-����д��FeS2�������ƺ�������Һ��Ϸ�Ӧ���ɶ�������(ClO2)�����ӷ���ʽ��______________________��
��3�� ��С�����ԡ�m(ClO2)/m(NaClO3)����Ϊ����ClO2���ʵ�ָ�ꡣ��ȡNaClO3��Ʒ6.0 g��ͨ����Ӧ�����ջ��400 mL ClO2��Һ��ȡ����Һ20 mL��37.00 mL 0.500 mol��L��1 (NH4)2Fe(SO4)2��Һ��ַ�Ӧ������Fe2������0.050 0 mol��L��1 K2Cr2O7����Һ�ζ����յ㣬����K2Cr2O7����Һ20.00 mL����Ӧԭ��Ϊ��
4H����ClO2��5Fe2��=Cl����5Fe3����2H2O
14H����Cr2O72-��6Fe2��=2Cr3����6Fe3����7H2O
�Լ���ClO2�ġ����ʡ�(д���������)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
д�����л�ѧ����ʽ�����ӷ���ʽ����뷽��ʽ��
��1��̼������Һ�����Ĵ�����Һ��Ӧ�����ӷ���ʽ��
��2��þ�ڶ�����̼������ȼ�գ���ѧ����ʽ��
��3����������루���뷽��ʽ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
���������ʣ��������Ȼ��� ������ �۴�����(CH3COOH) ��̼��� �ݾƾ� ������̼ ���������ƹ��� ��ͭ ��̼�����ƹ��������������Һ�����ڵ���ʵ��� �� ���ڷǵ���ʵ��� ���ܵ������ �����������գ�
��д�����ʢۺ͢���ˮ�з�Ӧ�����ӷ���ʽ�� ��
�ǽ����ʢ����Ƴ���Һ����ε��������Ba2+ǡ�ó�����ȫ��д�����ӷ���ʽ�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
1Lij�����Һ�����ܺ��е��������±���
| ���ܴ������е������� | H+��Ag+��Mg2+��Al3+��NH4+��Fe3+ |
| ���ܴ������е������� | Cl-��Br-��I-��CO32-��Al(OH)4-(��AlO2-) |

�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com