
��֪��Ӧ����1��10IkPaʱ��2C��S��+O2( g)=2CO(g) ��H=-221kJ/mol
��2����ϡ��Һ�У�H+��aq��+OH-��aq��=H2O��1������H=-57��3kJ/mol���н�����ȷ���� �� ��
A��̼��ȼ���ȴ���110��5kJ��mol
B����Ӧ��1���ķ�Ӧ��Ϊ221kJ��mol
C��ϡ�����ϡNaOH��Һ��Ӧ���к���Ϊ-57��3 kJ��mol
D��ϡ�����ϡNaOH��Һ��Ӧ����1 molˮ���ų�57��3kJ����

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
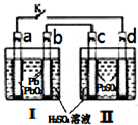 �ɻ�����������������̹�ˣ�������������Ϊ������Դ�ǵ��͵Ŀɳ��͵�أ��ܷ�ӦʽΪ��
�ɻ�����������������̹�ˣ�������������Ϊ������Դ�ǵ��͵Ŀɳ��͵�أ��ܷ�ӦʽΪ��
| ||
| ��� |
| ʵ������ | ʵ�鷽�� | ʵ������ԭ����� |
| �ٵ�����Ũ�������ƽ���Ӱ�� | ȡPbI2������Һ������һ֧�Թ��У��ټ�������NaI������Һ�� ȡPbI2������Һ������һ֧�Թ��У��ټ�������NaI������Һ�� |
��Һ�г��ֻ�ɫ���ǣ� ԭ������Һ��c��I-������ʹQc������pbI2��Ksp ��Һ�г��ֻ�ɫ���ǣ� ԭ������Һ��c��I-������ʹQc������pbI2��Ksp |
| ��Ǧ����Ũ�ȼ�С��ƽ���Ӱ�� | ȡPbI2����Һ������һ֧�Թ��У��ټ�������NaCl������Һ ȡPbI2����Һ������һ֧�Թ��У��ټ�������NaCl������Һ |
��ɫ������ʧ ԭ�����γ�PbCl42-��������Һ��c��Pb2+����С��ʹQcС����pbI2��Ksp ��ɫ������ʧ ԭ�����γ�PbCl42-��������Һ��c��Pb2+����С��ʹQcС����pbI2��Ksp |
| �� Ǧ���Ӻ͵�����Ũ�ȶ���С��ƽ���Ӱ�� Ǧ���Ӻ͵�����Ũ�ȶ���С��ƽ���Ӱ�� |
��PbI2����Һ�м�������FeCl3������Һ | PbI2 +2Fe3++4Cl-=PbCl42-+2Fe2++I2 PbI2 +2Fe3++4Cl-=PbCl42-+2Fe2++I2 |
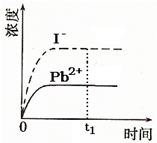 ����֪������PbI2��Ksp=8.0��10-9��������PbI2�������� 100mLˮ�����պñ��ͣ��ù�����Pb2+��I-Ũ����ʱ��仯��ϵ��ͼ������PbI2��Һ��c��I-��=0.0025mol?L-1������t1ʱ����������ϵ�м���100mL����0.020mol?L-1 NaI ��Һ������t1ʱ�̺�Pb2+��I-Ũ����ʱ��仯��ϵͼ��
����֪������PbI2��Ksp=8.0��10-9��������PbI2�������� 100mLˮ�����պñ��ͣ��ù�����Pb2+��I-Ũ����ʱ��仯��ϵ��ͼ������PbI2��Һ��c��I-��=0.0025mol?L-1������t1ʱ����������ϵ�м���100mL����0.020mol?L-1 NaI ��Һ������t1ʱ�̺�Pb2+��I-Ũ����ʱ��仯��ϵͼ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ���� |
| ���� |
| t/min | CH4��mol?L-1�� | H2O��mol?L-1�� | CO��mol?L-1�� | H2��mol?L-1�� |
| 0 | 0.2 | 0.3 | 0 | 0 |
| 2 | n1 | n2 | n3 | 0.3 |
| 3 | n1 | n2 | n3 | 0.3 |
| 4 | 0.09 | 0.19 | x | 0.33 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��13�֣������£�ijˮ��ҺM�д��ڵ������У�Na����A2����HA����H����OH�������ڵķ�����H2O��H2A����������ش��������⣺
(1)д����H2A�ĵ��뷽��ʽ_____________________________________________��
(2)����ҺM��10 mL 2 mol��L��1NaHA��Һ��2 mol��L��1NaOH��Һ�������϶��ã�����ҺM��pH________7(�>������<������)����Һ������Ũ���ɴ�С˳��Ϊ_____________________________________________��
��֪Ksp(BaA)��1.8��10��10����û����Һ�м���10 mL 1 mol��L��1BaCl2��Һ����Ϻ���Һ�е�Ba2��Ũ��Ϊ__________ mol��L��1��
(3)����ҺM�����������������0.01mol��L��1��H2A��Һ����0.01mol��L��1��NaHA��Һ����0.02mol��L��1��HCl��0.04 mol��L��1��NaHA��Һ��������Һ���������������Һ��H2A����Ũ������Ϊ________��pH�ɴ�С��˳��Ϊ____________��
(4)����ҺM��pH��3��H2A��ҺV1 mL��pH��11��NaOH��ҺV2 mL��Ϸ�Ӧ���ã������Һc(H��)/c(OH��)��104��V1��V2�Ĵ�С��ϵΪ____(����ڡ������ڡ���С�ڡ����п��ܡ�)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ��㶫ʡ��ͷ�н�ɽ��ѧ�߶�12���¿���ѧ�Ծ� ���ͣ������
��15�֣� �����£�ijˮ��ҺM�д��ڵ������У�Na����A2����HA����H����OH�������ڵķ�����H2O��H2A����������ش��������⣺
��1)д����H2A�ĵ��뷽��ʽ_____________________________________________��
��2��Na2A��ˮ��Һ��______�� ��ԭ���ǣ������ӷ���ʽ��ʾ����____________________
��Na2A��Һ�м��� ��������ˮ��
A NaOH���� B �Ȼ������� C ˮ D ̼���ƹ���
��֪Ksp(BaA)��1.8��10��10����20mL 1 mol��L��1Na2A��Һ�м���10 mL 1 mol��L��1BaCl2��Һ����Ϻ���Һ�е�Ba2��Ũ��Ϊ__________ mol��L��1��������A2����ˮ�⣩
(3)����ҺM��2 mol��L��1H2A��Һ��2mol��L��1NaOH��Һ�������϶��ã���������ҺM��pH<7������Һ������Ũ���ɴ�С˳��Ϊ_____________��
(4)����ҺM�����������������0.01 mol��L��1��H2A��Һ����0.01 mol��L��1��NaHA��Һ����0.02 mol��L��1��HCl��0.04 mol��L��1��NaHA��Һ��������Һ���������������Һ��H2A����Ũ������Ϊ________��pH�ɴ�С��˳��Ϊ____________��
��5������ҺM��pH��3��H2A��ҺV1 mL��pH��11��NaOH��ҺV2 mL��Ϸ�Ӧ���ã������Һc(H��)/c(OH��)��104��V1��V2�Ĵ�С��ϵΪ__________(����ڡ������ڡ���С�ڡ����п��ܡ�)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013��㶫ʡ��ͷ�и߶�12���¿���ѧ�Ծ� ���ͣ������
��15�֣� �����£�ijˮ��ҺM�д��ڵ������У�Na����A2����HA����H����OH�������ڵķ�����H2O��H2A����������ش��������⣺
��1)д����H2A�ĵ��뷽��ʽ_____________________________________________��
��2��Na2A��ˮ��Һ��______�� ��ԭ���ǣ������ӷ���ʽ��ʾ����____________________
��Na2A��Һ�м��� ��������ˮ��
A NaOH���� B �Ȼ������� C ˮ D ̼���ƹ���
��֪Ksp(BaA)��1.8��10��10����20mL 1 mol��L��1Na2A��Һ�м���10 mL 1 mol��L��1BaCl2��Һ����Ϻ���Һ�е�Ba2��Ũ��Ϊ__________ mol��L��1��������A2����ˮ�⣩
(3)����ҺM��2 mol��L��1H2A��Һ��2mol��L��1NaOH��Һ�������϶��ã���������ҺM��pH<7������Һ������Ũ���ɴ�С˳��Ϊ_____________��
(4)����ҺM�����������������0.01 mol��L��1��H2A��Һ����0.01 mol��L��1��NaHA��Һ����0.02 mol��L��1��HCl��0.04 mol��L��1��NaHA��Һ��������Һ���������������Һ��H2A����Ũ������Ϊ________��pH�ɴ�С��˳��Ϊ____________��
��5������ҺM��pH��3��H2A��ҺV1 mL��pH��11��NaOH��ҺV2 mL��Ϸ�Ӧ���ã������Һc(H��)/c(OH��)��104��V1��V2�Ĵ�С��ϵΪ__________(����ڡ������ڡ���С�ڡ����п��ܡ�)��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com