
��������AlN)�������¡�������������Ժõ��������ʣ����㷺���ڵ��ӹ�ҵ���մɹ�ҵ��������һ�������£���������ͨ�����·�Ӧ�ϳɣ�Al2O3+N2+3C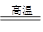 2AlN+3CO
2AlN+3CO
����������ȷ���ǣ�������
A���ڵ������ĺϳɷ�Ӧ�У�N2�ǻ�ԭ����Al2O3��������
B��������Ӧ��ÿ����2 mol AlN,N2�õ�3 mol����
C���������е�Ԫ�صĻ��ϼ�Ϊ-3
D���������������ڷ��Ӿ���
C
��������
������������ݷ���ʽ��֪����Ԫ�صĻ��ϼ۴�0�۽��͵���3�ۣ��õ�3�����ӣ�����ԭ������������̼Ԫ�صĻ��ϼ۴�0�����ߵ���2�ۣ�ʧȥ2�����ӣ�������������ԭ��������A��B����ȷ��C��ȷ������AlN�����ʿ��Ʋ⣬�������γɵľ��������Ӿ��壬D����ȷ����ѡC��
���㣺����������ԭ��Ӧ���й��жϡ����㡢���ϼ۵��ж��Լ��������͵��ж�
�������������е��Ѷȵ����⣬Ҳ�Ǹ߿��еij������͡����������߿���������ǿ�������������С�����������ѧ����˼ά������Ҳ���������ѧ���������⡢��������������


 �㾦�½̲�ȫ�ܽ��ϵ�д�
�㾦�½̲�ȫ�ܽ��ϵ�д� Сѧ�̲���ȫ���ϵ�д�
Сѧ�̲���ȫ���ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�


| ||||
|
| ||||
|
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
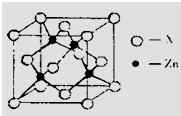 Zn��Al���������ԣ��䵥�ʺͻ������ڽ���ҵ�����ӹ�ҵ��ʯ�ͻ����ȷ���Ӧ�ù㷺����ش��������⣺
Zn��Al���������ԣ��䵥�ʺͻ������ڽ���ҵ�����ӹ�ҵ��ʯ�ͻ����ȷ���Ӧ�ù㷺����ش��������⣺�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com