
”¾ĢāÄæ”æĢ¼ŗĶµŖŹĒ¶ÆÖ²ĪļĢåÖŠµÄÖŲŅŖ×é³ÉŌŖĖŲ£¬Ļņ“óĘųÖŠ¹ż¶ČÅŷŶžŃõ»ÆĢ¼»įŌģ³ÉĪĀŹŅŠ§Ó¦£¬µŖŃõ»ÆĪļ»į²śÉś¹ā»ÆѧŃĢĪķ£¬ÄæĒ°£¬ÕāŠ©ÓŠ¶¾ÓŠŗ¦ĘųĢåµÄ“¦Ąķ³ÉĪŖæĘѧъ¾æµÄÖŲŅŖÄŚČŻ”£
£Ø1£©ŅŃÖŖČČ»Æѧ·½³ĢŹ½£ŗ¢Ł2C2H2(g)£«5O2(g)===4CO2(g)£«2H2O(l)””¦¤H1
¢ŚC(s)£«O2(g)===CO2(g)””¦¤H2
¢ŪH2(g)£«1/2O2(g)===H2O(l)””¦¤H3
Ōņ·“Ó¦¢Ü2C(s)£«H2(g)===C2H2(g)µÄ¦¤H=_________”£(ÓĆŗ¬¦¤H1”¢¦¤H2”¢¦¤H3µÄ¹ŲĻµŹ½±ķŹ¾)
£Ø2£©ĄūÓĆÉĻŹö·“Ó¦¢ŁÉč¼ĘČ¼ĮĻµē³Ų£Øµē½āÖŹČÜŅŗĪŖĒāŃõ»Æ¼ŲČÜŅŗ£©£¬Š“³öµē³ŲµÄøŗ¼«·“Ó¦Ź½£ŗ__________________________________________ ”£
£Ø3£©ÓĆ»īŠŌĢ滹Ō·ØæÉŅŌ“¦ĄķµŖŃõ»ÆĪļ”£Ä³ŃŠ¾æŠ”×éĻņijĆܱÕČŻĘ÷ÖŠ¼ÓČėŅ»¶ØĮæµÄ»īŠŌĢæŗĶNO£¬·¢Éś·“Ó¦C(s)£«2NO(g)![]() N2(g)£«CO2(g)””¦¤H<0”£ŌŚT1”ꏱ£¬·“Ó¦½ųŠŠµ½²»Ķ¬Ź±¼ä²āµĆø÷ĪļÖŹµÄÅضČČēĻĀ£ŗ
N2(g)£«CO2(g)””¦¤H<0”£ŌŚT1”ꏱ£¬·“Ó¦½ųŠŠµ½²»Ķ¬Ź±¼ä²āµĆø÷ĪļÖŹµÄÅضČČēĻĀ£ŗ
Ź±¼ä/min ÅضČ/(mol”¤L£1) ĪļÖŹ | 0 | 10 | 20 | 30 | 40 | 50 |
NO | 1.00 | 0.68 | 0.50 | 0.50 | 0.60 | 0.60 |
N2 | 0 | 0.16 | 0.25 | 0.25 | 0.30 | 0.30 |
CO2 | 0 | 0.16 | 0.25 | 0.25 | 0.30 | 0.30 |
¢Ł10”«20 minÄŚ£¬NOµÄĘ½¾ł·“Ó¦ĖŁĀŹv(NO)£½______£¬T1”ꏱ£¬øĆ·“Ó¦µÄĘ½ŗā³£ŹżK£½________”£
¢Ś30 minŗó£¬Ö»øıäijŅ»Ģõ¼ž£¬·“Ó¦ÖŲŠĀ“ļµ½Ę½ŗā£¬øł¾ŻÉĻ±ķÖŠµÄŹż¾ŻÅŠ¶ĻøıäµÄĢõ¼žæÉÄÜŹĒ________(Ģī×ÖÄø±ąŗÅ)”£
a£®ĶØČėŅ»¶ØĮæµÄNO b£®¼ÓČėŅ»¶ØĮæµÄC c£®ŹŹµ±Éżøß·“Ó¦ĢåĻµµÄĪĀ¶Č
d£®¼ÓČėŗĻŹŹµÄ“߻ƼĮ e£®ŹŹµ±ĖõŠ”ČŻĘ÷µÄĢå»ż
¢ŪČō±£³ÖÓėÉĻŹö·“Ó¦Ē°30 minµÄ·“Ó¦Ģõ¼žĻąĶ¬£¬ĘšŹ¼Ź±NOµÄÅضČĪŖ2.50 mol”¤L£1£¬Ōņ·“Ó¦“ļĘ½ŗāŹ±c(NO)£½________£¬NOµÄ×Ŗ»ÆĀŹ£½________”£
”¾“š°ø”æ2¦¤H2£«¦¤H3£1/2¦¤H1 C2H2£10e££«14OH£==2CO32-£«8H2O 0.018 mol”¤L£1”¤min£1 0.25 ae 1.25 mol”¤L£1 50%
”¾½āĪö”æ
øł¾ŻøĒĖ¹¶ØĀɼĘĖć·“Ó¦ČČ£»øł¾ŻČ¼ĮĻµē³ŲµÄ×Ü·“Ó¦ŗĶČ¼ÉÕ·“Ó¦²śĪļĻąĖʵÄĢŲÕ÷·ÖĪöŹéŠ“µē¼«·½³ĢŹ½£»øł¾ŻĘ½ŗā³£Źż¼ĘĖćĻą¹ŲĪļÖŹµÄÅØ¶Č”£
(1)øł¾ŻøĒĖ¹¶ØĀÉ£¬·“Ó¦²»ĀŪŹĒŅ»²½Ķź³ÉµÄ»¹ŹĒ¼ø²½Ķź³ÉµÄ£¬Ęä·“Ó¦ČȵÄ×ÜÖµĻąµČ£¬½«·“Ó¦¢ŚĄ©“óĮ½±¶ŌŁ¼ÓÉĻ·“Ó¦¢Ū£¬ŌŁ¼õČ„1/2·“Ó¦¢Ł¾ĶæÉŅŌµĆµ½·“Ó¦¢Ü£¬ĖłŅŌ·“Ó¦¢ÜµÄ·“Ó¦ČČĪŖ£ŗ2¦¤H2£«¦¤H3£1/2¦¤H1£»
(2)Č¼ĮĻµē³ŲµÄµē½āÖŹĪŖĒāŃõ»Æ¼Ų£¬Ōņ²śĪļĪŖĢ¼Ėįøł£¬øŗ¼«Ź§µē×Ó£¬·¢ÉśŃõ»Æ·“Ó¦£¬½įŗĻČ¼ÉÕµÄ×Ü·“Ó¦·½³ĢŹ½µĆ£ŗC2H2£10e££«14OH£==2CO32-£«8H2O£»
(3)ÓɱķÖŠŹż¾ŻÖŖ¢Ł10min”«20minÄŚ£¬NOÅØ¶ČµÄ±ä»ÆĪŖ0.68 mol”¤L£1µ½0.50 mol”¤L£1
v(NO)=( 0.68 mol”¤L£1-0.50 mol”¤L£1)/10min=0.018 mol”¤L£1”¤min£1£»
20minŗó“ļµ½»ÆŃ§Ę½ŗāדĢ¬£¬ÓÉÓŚCĪŖ¹ĢĢ壬»ÆŃ§Ę½ŗā³£Źż±ķ“ļŹ½ĪŖ![]() =(0.25”Į0.25)/0.5”Į0.5=0.25£»
=(0.25”Į0.25)/0.5”Į0.5=0.25£»
¢Ś30 minŗ󣬓ӱķÖŠæÉŅŌ擳öÅØ¶Č¶¼Ōö“óĮĖ£¬a£®ĶØČėŅ»¶ØĮæµÄNO£¬Ę½ŗāĻņÕż·½ĻņŅĘ¶Æ£¬·ūŗĻĢāŅā£» b£®¼ÓČėŅ»¶ØĮæµÄC£¬ŅņĪŖCĪŖ¹ĢĢ¬£¬¹Ź¶ŌĘ½ŗā²»Ó°Ļģ£¬²»·ūŗĻĢāŅā£»c£®ŹŹµ±Éżøß·“Ó¦ĢåĻµµÄĪĀ¶Č£¬·“Ó¦¦¤H<0£¬Ę½ŗāĻņÄę·½ĻņŅĘ¶Æ£¬²»·ūŗĻĢāŅā£»d£®¼ÓČėŗĻŹŹµÄ“߻ƼĮ£¬Ę½ŗā²»ŅĘ¶Æ£¬²»·ūŗĻĢāŅā£»e£®ŹŹµ±ĖõŠ”ČŻĘ÷µÄĢå»ż£¬·“Ó¦Ē°ŗóĘųĢå×ÜĪļÖŹµÄĮæĻąĶ¬£¬Ę½ŗā²»ŅĘ¶Æ£¬µ«ÅØ¶Č»įŌö“󣬷ūŗĻĢāŅā£¬¹Ź“š°øĪŖ£ŗae£»
¢Ū Ę½ŗā³£ŹżÖ»ÓėĪĀ¶ČÓŠ¹Ų£¬ŌņK=0.25£¬Éčc(NO)=x
C(s)£«2NO(g) ![]() N2(g) £« CO2(g)
N2(g) £« CO2(g)
Ź¼£Ømol”¤L£1£© 2.50 0 0
±ä£Ømol”¤L£1£© 2.5-x (2.5-x)/2 (2.5-x)/2
Ę½£Ømol”¤L£1£© x (2.5-x)/2 (2.5-x)/2
K=![]() =[(2.5-x)/2]2/x2=0.25£¬x=1.25 mol”¤L£1£»×Ŗ»ÆĀŹĪŖ£ŗ
=[(2.5-x)/2]2/x2=0.25£¬x=1.25 mol”¤L£1£»×Ŗ»ÆĀŹĪŖ£ŗ![]() ”Į100%=50%”£
”Į100%=50%”£


 100·Ö“³¹ŲĘŚÄ©³å“ĢĻµĮŠ“š°ø
100·Ö“³¹ŲĘŚÄ©³å“ĢĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æÓÉC”¢H”¢OŌŖĖŲ×é³ÉµÄ¾ßÓŠ¹ūĻćĪ¶µÄŅŗĢåA£ØC4H8O2£©£¬æÉ·¢ÉśĻĀĶ¼ĖłŹ¾µÄ×Ŗ»Æ£ŗ
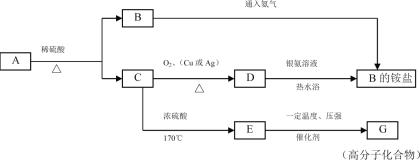
£Ø1£©Š“³öĻĀĮŠĪļÖŹµÄ½į¹¹¼ņŹ½A_____________£¬D______________£¬G_____________”£
£Ø2£©ŌŚC”śEµÄ·“Ó¦ÖŠ£¬ÅØĮņĖįµÄ×÷ÓĆŹĒ_______”¢_______”£
£Ø3£©×¢Ć÷C”śE·“Ó¦µÄĄąŠĶ_________________”£
£Ø4£©ŹŌÓĆŹµŃéÖ¤Ć÷EæÉŅŌ·¢Éś¼Ó³É·“Ó¦£¬²¢Š“·“Ó¦µÄ»Æѧ·½³ĢŹ½_____________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”涬ĒąÓĶÓÖ½ŠĖ®ŃīĖį¼×õ„£¬ŹĒŅ»ÖÖÖŲŅŖµÄÓŠ»śŗĻ³ÉŌĮĻ”£Ä³»ÆѧŠ”×éÓĆĖ®ŃīĖį(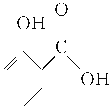 )ŗĶ¼×“¼ŌŚĖįŠŌ“߻ƼĮ“ß»ÆĻĀŗĻ³ÉĖ®ŃīĖį¼×õ„²¢¼ĘĖćĘä²śĀŹ”£
)ŗĶ¼×“¼ŌŚĖįŠŌ“߻ƼĮ“ß»ÆĻĀŗĻ³ÉĖ®ŃīĖį¼×õ„²¢¼ĘĖćĘä²śĀŹ”£
ŹµŃé²½Öč£ŗ
¢ń.ČēĶ¼£¬ŌŚČż¾±ÉÕĘæÖŠ¼ÓČė13.8 g(0.1 mol)Ė®ŃīĖįŗĶ24 g(30 mL,0.75 mol)¼×“¼£¬Ļņ»ģŗĻĪļÖŠ¼ÓČėŌ¼10 mL¼×±½(¼×±½ÓėĖ®ŠĪ³É¹²·ŠĪļ£¬·ŠµćĪŖ85 ”ę£¬øĆŹµŃéÖŠ¼ÓČė¼×±½£¬Ņ×½«Ė®Õō³ö)£¬ŌŁŠ”ŠÄµŲ¼ÓČė5 mLÅØĮņĖį£¬Ņ”¶Æ»ģŌČ£¬¼ÓČė1”«2Į£·ŠŹÆ£¬×é×°ŗĆŹµŃé×°ÖĆ£¬ŌŚ85”«95 ”ęĻĀŗćĪĀ¼ÓČČ·“Ó¦1.5Š”Ź±£»
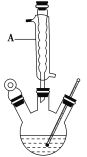
¢ņ.“ż×°ÖĆĄäČ“ŗó£¬·ÖĄė³ö¼×“¼£¬Č»ŗó×ŖŅĘÖĮ·ÖŅŗĀ©¶·£¬ŅĄ“ĪÓĆÉŁĮæĖ®”¢5% NaHCO3ČÜŅŗŗĶĖ®Ļ“µÓ£»·ÖĄė³öµÄ²śĪļ¼ÓČėÉŁĮæĪŽĖ®MgSO4¹ĢĢ壬¹żĀĖµĆµ½“Öõ„£»
¢ó.½«“Öõ„½ųŠŠÕōĮó£¬ŹÕ¼Æ221”«224 ”ęµÄĮó·Ö£¬µĆĖ®ŃīĖį¼×õ„9.12 g”£
³£ÓĆĪļĄķ³£Źż£ŗ
Ćū³Ę | ·Ö×ÓĮæ | ŃÕɫדĢ¬ | Ļą¶ŌĆÜ¶Č (g”¤cm£3) | ČŪµć (”ę) | ·Šµć (”ę) |
Ė®ŃīĖį ¼×õ„ | 152 | ĪŽÉ«ŅŗĢå | 1.18 | £8.6 | 218”« 224 |
Ė®ŃīĖį | 138 | °×É«¾§Ģå | 1.44 | 158 | 210 |
¼×“¼ | 32 | ĪŽÉ«ŅŗĢå | 0.792 | £97 | 64.7 |
Ēėøł¾ŻŅŌÉĻŠÅĻ¢»Ų“šĻĀĮŠĪŹĢā£ŗ
£Ø1£©ŅĒĘ÷AµÄĆū³ĘŹĒ________£¬¼ÓČė·ŠŹÆµÄ×÷ÓĆŹĒ_____________________”£Čō¼ÓČČŗó·¢ĻÖĪ“¼Ó·ŠŹÆ£¬Ó¦²ÉČ”µÄÕżČ··½·ØŹĒ________________________________________________”£
£Ø2£©ÖʱøĖ®ŃīĖį¼×õ„Ź±£¬×īŗĻŹŹµÄ¼ÓČČ·½·ØŹĒ____________________________________________”£
£Ø3£©ŹµŃéÖŠ¼ÓČė¼×±½¶ŌŗĻ³ÉĖ®ŃīĖį¼×õ„µÄ×÷ÓĆŹĒ___________________________________________”£
£Ø4£©·“Ó¦½įŹųŗó£¬·ÖĄė³ö¼×“¼²ÉÓƵķ½·ØŹĒ__________________________________________”£
£Ø5£©ŹµŃéÖŠ¼ÓČėĪŽĖ®ĮņĖįĆ¾µÄ×÷ÓĆŹĒ__________________________________________”£±¾ŹµŃéµÄ²śĀŹĪŖ________(±£ĮōĮ½Ī»ÓŠŠ§Źż×Ö)”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æČēĶ¼ŹĒ³£¼ūŌµē³Ų×°ÖĆ£¬µēĮ÷±ķA·¢ÉśĘ«×Ŗ”£
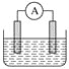
(1)ČōĮ½øöµē¼«·Ö±šŹĒŠæ”¢Ķ£¬µē½āÖŹČÜŅŗŹĒĻ”ĮņĖį£¬Õż¼«µÄµē¼«·“Ó¦Ź½ĪŖ_________________£»Čē¹ū°Ńµē½āÖŹČÜŅŗ»»³ÉĮņĖįĶČÜŅŗ£¬ŌņÕż¼«µÄµē¼«·“Ó¦Ź½ĪŖ____________________________”£
(2)Čōµē³ŲµÄ×Ü·“Ó¦ŹĒ2FeCl3£«Fe![]() 3FeCl2£¬ŌņæÉŅŌ×÷øŗ¼«²ÄĮĻµÄŹĒ________£¬øŗ¼«·“Ó¦Ź½ŹĒ____________________________£¬Õż¼«·“Ó¦Ź½ŹĒ_________________________________”£
3FeCl2£¬ŌņæÉŅŌ×÷øŗ¼«²ÄĮĻµÄŹĒ________£¬øŗ¼«·“Ó¦Ź½ŹĒ____________________________£¬Õż¼«·“Ó¦Ź½ŹĒ_________________________________”£
(3)Čōµē½āÖŹČÜŅŗŹĒĻ”ĮņĖį£¬Ć¾”¢ĀĮĮ½ÖÖ½šŹō×÷µē¼«£¬ŌņĆ¾µē¼«µÄ·“Ó¦Ź½ĪŖ_________________£»Čōµē½āÖŹČÜŅŗ»»×÷Ļ”ĒāŃõ»ÆÄĘČÜŅŗ£¬Ć¾”¢ĀĮĮ½½šŹō×÷µē¼«£¬ŌņĆ¾ŹĒ________(Ń”Ģī”°Õż¼«”±»ņ”°øŗ¼«”±)£¬×Ü·“Ó¦µÄĄė×Ó·½³ĢŹ½ŹĒ_________________________________________________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æijŠ£ŠĖȤŠ”×é¶ŌĒāŃõ»ÆÄĘČÜŅŗŗĶĻ”ŃĪĖį»ģŗĻŗóµÄÓŠ¹ŲĪŹĢā£¬½ųŠŠĮĖČēĻĀĢ½¾æ£ŗ
(1)¢Ł¼×Ķ¬Ń§ĪŖĮĖÖ¤Ć÷ĒāŃõ»ÆÄĘČÜŅŗÓėĻ”ŃĪĖįÄܹ»·¢Éś·“Ó¦£¬Éč¼Ę²¢Ķź³ÉĮĖČēĻĀĶ¼ĖłŹ¾ŹµŃé£ŗ
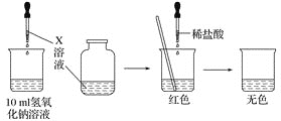
XČÜŅŗŹĒ________£¬µĪČėµÄĮæŅ»°ćĪŖ________”£
¢ŚŅŅĶ¬Ń§ČĻĪŖ¼×Ķ¬Ń§µÄŹµŃé²»Äܳä·ÖÖ¤Ć÷ĒāŃõ»ÆÄĘČÜŅŗÓėĻ”ŃĪĖįÄܹ»·¢Éś·“Ó¦£¬ŅŅĶ¬Ń§µÄĄķÓÉŹĒ________________________________________________________________________”£
(2)±ūĶ¬Ń§ĄūÓĆ”°±£ĪĀĘæŹ½ĮæČČ¼Ę”±£¬²ā³ö10 mL 10%ĒāŃõ»ÆÄĘČÜŅŗŗĶ²»Ķ¬Ģå»żµÄ10%ŃĪĖį»ģŗĻ¹ż³ĢÖŠ£¬ČÜŅŗµÄĪĀ¶Č±ä»Æ£¬¼ūĻĀ±ķ(¼ŁÉčĮ½ČÜŅŗĆܶČĻąĶ¬)”£
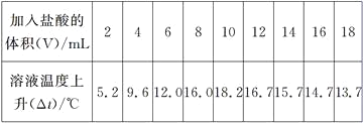
¾Ķ“ĖŹµŃé»Ų“š£ŗ
¢ŁŃĪĖįŗĶĒāŃõ»ÆÄʵķ“Ó¦ŹĒ________(Ģī”°ĪüČČ”±»ņ”°·ÅČČ”±)·“Ó¦”£
¢ŚĒėŌŚĻĀĶ¼ÖŠ»ęÖĘ³öČÜŅŗµÄĪĀ¶ČÉĻÉżÓė¼ÓČėŃĪĖįĢå»żÖ®¼äµÄ±ä»Æ¹ŲĻµĶ¼______”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æŗČ²č£¬¶ŌŗܶąČĖĄ“ĖµŹĒČĖÉśµÄŅ»“óæģĄÖ£¬²čŅ¶ÖŠŗ¬ÓŠÉŁĮæµÄ槷ČŅņ”£æ§·ČŅņ¾ßÓŠĄ©ÕÅŃŖ¹Ü”¢“Ģ¼¤ŠÄÄŌµČ×÷ÓĆ£¬ŌŚ100 ”ꏱŹ§Č„½į¾§Ė®²¢æŖŹ¼Éż»Ŗ£¬120 ”ꏱɿ»ŖĻąµ±ĻŌÖų£¬178 ”ꏱɿ»ŖŗÜæģ”£½į¹¹¼ņŹ½ČēĻĀ£ŗ
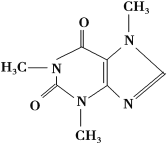
ŹµŃéŹŅæÉĶعżĻĀĮŠ¼ņµ„·½·Ø“Ó²čŅ¶ÖŠĢįČ”æ§·ČŅņ£ŗ

(1)槷ČŅņµÄ·Ö×ÓŹ½ĪŖ___________________________________________”£
(2)²½Öč1½žÅŻ²čŅ¶ĖłÓƵÄČܼĮ×īŗĆĪŖ________”£
A£®Ė®”” B£®¾Ę¾«””””””””C£®ŹÆÓĶĆŃ
(3)²½Öč1”¢²½Öč4Ėł½ųŠŠµÄ²Ł×÷»ņ·½·Ø·Ö±šŹĒ________£¬________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æĖ®µÄÓ²¶Č¹żø߶Ō¹¤ŅµÉś²śÓŠŃĻÖŲĪ£ŗ¦£¬æɽµµĶĖ®µÄÓ²¶ČµÄ·½·ØŹĒ( )
A.¹żĀĖ·ØB.ÖŠŗĶ·ØC.Ąė×Ó½»»»·ØD.Ć÷·Æ¾»»Æ·Ø
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æČēĶ¼ŹĒÓÉ4øöĢ¼Ō×Ó½įŗĻ³ÉµÄ6ÖÖÓŠ»śĪļ(ĒāŌ×Óƻӊ»³ö)
![]()
(1) Š“³öÓŠ»śĪļ(a)µÄĻµĶ³ĆüĆū·ØµÄĆū³Ę___________________”£
(2) ÓŠ»śĪļ(a)ÓŠŅ»ÖÖĶ¬·ÖŅģ¹¹Ģ壬ŹŌŠ“³öĘä½į¹¹¼ņŹ½__________________”£
(3) ÉĻŹöÓŠ»śĪļÖŠÓė(c)»„ĪŖĶ¬·ÖŅģ¹¹ĢåµÄŹĒ________(Ģī“śŗÅ)”£
(4) ČĪŠ“Ņ»ÖÖÓė(e)»„ĪŖĶ¬ĻµĪļµÄÓŠ»śĪļµÄ½į¹¹¼ņŹ½____________”£
(5) ÉĻŹöÓŠ»śĪļÖŠ²»ÄÜÓėäå·“Ó¦²¢Ź¹ĘäĶŹÉ«µÄÓŠ________(Ģī“śŗÅ)”£
(6) (a)(b)(c)(d)ĖÄÖÖĪļÖŹÖŠ£¬4øöĢ¼Ō×ÓŅ»¶Ø“¦ÓŚĶ¬Ņ»Ę½ĆęµÄÓŠ________(Ģī“śŗÅ)”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æ»ÆŗĻĪļXŹĒŅ»ÖÖĻćĮĻ£¬æɲÉÓĆŅŅĻ©Óė¼×±½ĪŖÖ÷ŅŖŌĮĻ£¬°“ĻĀĮŠĀ·ĻßŗĻ³É£ŗ
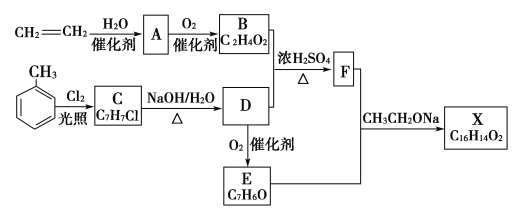
(1)Š“³öÓÉŅŅĻ©ÖĘČ”AµÄ»Æѧ·½³ĢŹ½£ŗ______________________________________________________”£
(2)ŅŅĻ©ÄÜŹ¹äåĖ®ŗĶĖįŠŌKMnO4ČÜŅŗĶŹÉ«£¬¶žÕßĶŹÉ«ŌĄķĻąĶ¬Āš£æ________”£ŌŅņŹĒ_____________________________________________________________________”£
(3)ŅŌŅŅĻ©ĪŖŌĮĻ£¬ÄÜ·ńÖʵĆŅŅČ²£æ________”£ČōÄÜ£¬ĒėŠ“³öĻą¹ŲµÄ»Æѧ·½³ĢŹ½£ŗ_________________________________________________________________________________”£
(4)ĒėŠ“³öCµÄŗ¬ÓŠ±½»·µÄĶ¬·ÖŅģ¹¹ĢåµÄ½į¹¹¼ņŹ½£ŗ___________________________________”£
(5)Š“³ö¼×±½ÓėÅØĻõĖįŗĶÅØĮņĖįµÄ»ģŗĻĖį·“Ó¦µÄ»Æѧ·½³ĢŹ½£ŗ________________________”£
(6)ŅŌ¼×±½ĪŖĄżĖµĆ÷ÓŠ»śĪļ»łĶÅÖ®¼äµÄĻą»„Ó°Ļģ£ŗ_______________________”£
(7)Š“³öC”śDµÄ»Æѧ·½³ĢŹ½£ŗ__________________________________________£¬Ęä·“Ó¦ĄąŠĶĪŖ____________________________________”£
(8)CÄÜ·¢ÉśĻūČ„·“Ó¦Āš£æ________”£ŌŅņŹĒ__________________________________________”£
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com