
ijͬѧ������ͼ��ʾ��ʵ��װ�ý�������ˮ������Ӧ��ʵ�飬�������о������仯����IJ������ʡ�

��ش��������⣺
��1��Ӳ���Թ��з�����Ӧ�Ļ�ѧ����ʽΪ____________________________ ��
��2����ͬѧ��ȷ����Ӧ��Ӳ���Թ��й������ʵijɷ֣����������ʵ�鷽����
�ٴ�Ӳ���Թ���ȴ��ȡ�������еĹ�����������ϡ�������ҺB��
��ȡ������ҺB�μ�KSCN��Һ������Һ���ɫ��˵��Ӳ���Թ��й������ʵijɷ�һ���� �������� ������Һδ���ɫ��˵��Ӳ���Թ��й������ʵijɷ��� ��
��3����ͬѧ������ʵ�鷽��������ʵ�飬�����Һδ���ɫ��ԭ���ǣ�
�������ӷ���ʽ��ʾ����
��4����ͬѧ������ȡ������ҺB��ʹ���NaOH��Һ��Ӧ��������ͼ��ʾ�IJ������ɹ۲쵽���ɰ�ɫ������Ѹ�ٱ�ɻ���ɫ������ɺ��ɫ��������д��������ɫ������ɺ��ɫ�Ļ�ѧ����ʽ�� ��

��5��һ��ʱ���ͬѧ���֣�3����δ������Һ��ɺ�ɫ��˵��Fe2+ ���� �ԡ��ɴ˿�֪��ʵ�����к�Fe2+������Һ���������Ƶ�ԭ���� ���������ƺ�Fe2+������ҺʱӦ�������� ��
��1��3Fe + 4H2O(g) �� Fe3O4 + 4H2��2�֣�
��2��һ����Fe3O4��������Fe����1�֣� Fe3O4��Fe��1�֣�
��3��Fe + 2Fe3+�� 3Fe2+��2�֣�
��4�� 4Fe(OH)2 + O2 + 2H2O �� 4Fe(OH)3��2�֣�
��5����ԭ Fe2+�ױ������е��������������� ���� ����1�֣�
��������
�����������1��Ӳ���Թ��з�����Ӧ������ˮ�����ķ�Ӧ��ע����ֻ����������������������������д����������������2������Һ���ɫ��˵����Һ�������������ӣ������ų���Һ���Ƿ����������ӣ�����Ӳ���Թ��й������ʵijɷ�һ����Fe3O4,������Fe������Һδ���ɫ��˵����Һ�������������ӣ���һ���й�����������������ԭΪ���������ӣ�Ӳ���Թ��й������ʵijɷ�һ����Fe3O4��Fe����3��˵��������������������ԭΪ��������Fe + 2Fe3+�� 3Fe2+����4�����ɰ�ɫ����������������,Ѹ�ٱ�ɻ���ɫ,����ɺ��ɫ����������������������Ϊ��������, FeSO4 + 2NaOH �� Fe(OH)2��+ Na2SO4�� 4Fe(OH)2 + O2 + 2H2O �� 4Fe(OH)3����5��δ������Һ��ɺ�ɫ��Fe2+������Ϊ���������ӣ�˵�����л�ԭ�ԡ�ʵ�����к�Fe2+������Һ�����������Ƶ�ԭ�����ױ������е��������������ʣ��������ƺ�Fe2+������ҺʱӦ�����������ۣ�������������������ٻ�ԭ���������ӡ�
���㣺��������ˮ������Ӧ��ʵ�飬������������������ķ������������������Ʊ��������ǵ�ת����
���������������ʼ��仯�����ת���Ǹ��е��ص���ѵ㣬���������ӵļ��顢���ӡ����ʵ��Ʊ������ͳ��֣��Ƚ��ۺϣ�����ѧ�����ۺ�˼ά��������Ŀ�ѶȽϴ�


 ��ĩ���100�ִ��½����ȫ�Ծ�ϵ�д�
��ĩ���100�ִ��½����ȫ�Ծ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ��ɽ��ʡ��������ˮһ�и�һ��ѧ����ĩģ�⻯ѧ�Ծ����������� ���ͣ�ʵ����
(17��)ijͬѧ������ͼ��ʾ��ʵ��װ�ý�������ˮ������Ӧ��ʵ��,�������о������仯����IJ������ʡ�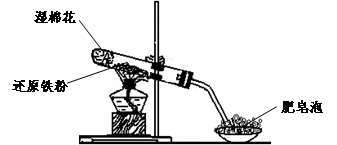
��֪:��FeO + 2H+ = Fe2+ + H2O��Fe2O3 + 6H+ = 2Fe3+ +3 H2O ��Fe3O4 + 8H+ = Fe2+ +2Fe3+ +4 H2O
��ش���������:
��1��Ӳ���Թ��з�����Ӧ�Ļ�ѧ����ʽΪ ��
��2����ͬѧ��ȷ����Ӧһ��ʱ���Ӳ���Թ��й������ʵijɷ�,���������ʵ�鷽��:
�ٴ�Ӳ���Թ���ȴ��,ȡ�������еĹ�����������ϡ�������ҺB;
��ȡ������ҺB�μ�KSCN��Һ,����Һ���ɫ��˵��Ӳ���Թ��й������ʵijɷ���(ֻ��һ��ѡ���������) ,����Һδ���ɫ��˵��Ӳ���Թ��й������ʵijɷ���(ֻ��һ��ѡ���������) ��
| A��һ����Fe3O4,������Fe | B��ֻ��Fe(OH)3 | C��һ����Fe3O4��Fe |
| D��һ����Fe(OH)3,������Fe E.ֻ��Fe3O4 |


�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ���Ĵ�ʡ�ɶ����и�һ��ѧ��2��ѧҵ��⻯ѧ�Ծ� ���ͣ�ʵ����
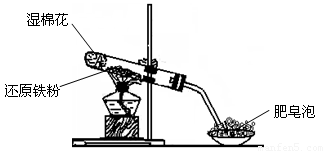
��13�֣�ijͬѧ������ͼ��ʾ��ʵ��װ�ý�������ˮ������Ӧ��ʵ�飬�������о������仯����IJ������ʡ�
��ش��������⣺
��1��Ӳ���Թ��з�����Ӧ�Ļ�ѧ����ʽΪ____________________________ ��
��2����ͬѧ��ȷ����Ӧ��Ӳ���Թ��й������ʵijɷ֣����������ʵ�鷽����
�ٴ�Ӳ���Թ���ȴ��ȡ�������еĹ�����������ϡ�������ҺB��
��ȡ������ҺB�μ�KSCN��Һ������Һ���ɫ��˵��Ӳ���Թ��й������ʵijɷ�һ���� �������� ������Һδ���ɫ��˵��Ӳ���Թ��й������ʵijɷ��� ��
��3����ͬѧ������ʵ�鷽��������ʵ�飬�����Һδ���ɫ��ԭ���ǣ�
�������ӷ���ʽ��ʾ����
��4����ͬѧ������ȡ������ҺB��ʹ���NaOH��Һ��Ӧ������ͼ��ʾ�IJ������ɹ۲쵽���ɰ�ɫ������Ѹ�ٱ�ɻ���ɫ������ɺ��ɫ��������д��������ɫ������ɺ��ɫ�Ļ�ѧ����ʽ�� 
��5��һ��ʱ���ͬѧ���֣�3����δ������Һ��ɺ�ɫ��
˵��Fe2+ ���� �ԡ��ɴ˿�֪��ʵ�����к�Fe2+������Һ���������Ƶ�ԭ���� ���������ƺ�Fe2+������ҺʱӦ�������� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014���Ĵ�ʡ��һ��ѧ��2��ѧҵ��⻯ѧ�Ծ� ���ͣ�ʵ����
��13�֣�ijͬѧ������ͼ��ʾ��ʵ��װ�ý�������ˮ������Ӧ��ʵ�飬�������о������仯����IJ������ʡ�
��ش��������⣺
��1��Ӳ���Թ��з�����Ӧ�Ļ�ѧ����ʽΪ____________________________ ��
��2����ͬѧ��ȷ����Ӧ��Ӳ���Թ��й������ʵijɷ֣����������ʵ�鷽����
�ٴ�Ӳ���Թ���ȴ��ȡ�������еĹ�����������ϡ�������ҺB��
��ȡ������ҺB�μ�KSCN��Һ������Һ���ɫ��˵��Ӳ���Թ��й������ʵijɷ�һ���� �������� ������Һδ���ɫ��˵��Ӳ���Թ��й������ʵijɷ��� ��
��3����ͬѧ������ʵ�鷽��������ʵ�飬�����Һδ���ɫ��ԭ���ǣ�
�������ӷ���ʽ��ʾ����
��4����ͬѧ������ȡ������ҺB��ʹ���NaOH��Һ��Ӧ������ͼ��ʾ�IJ������ɹ۲쵽���ɰ�ɫ������Ѹ�ٱ�ɻ���ɫ������ɺ��ɫ��������д��������ɫ������ɺ��ɫ�Ļ�ѧ����ʽ��
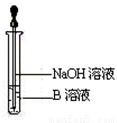
��5��һ��ʱ���ͬѧ���֣�3����δ������Һ��� ��ɫ��
˵��Fe2+ ���� �ԡ��ɴ˿�֪��ʵ�����к�Fe2+������Һ���������Ƶ�ԭ���� ���������ƺ�Fe2+������ҺʱӦ�������� ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com