
| ��� | ���� | ʵ������ |
| �� | �ֱ����Թ�A��B�м���3mL 5% H2O2��Һ��������2��1mol/L FeCl3��Һ�����Թ��о����������ݳ���ʱ�����Թ�A����ʢ��5��������ˮ���ձ��н��ݣ����Թ�B����ʢ��40��������ˮ���ձ��н��ݣ� | �Թ�A�в��ٲ������ݣ� �Թ�B�в��������������� |
| �� | ��ȡ��֧�Թֱܷ����3mL 5% H2O2��Һ��3mL 10% H2O2��Һ | �Թ�A��B�о�δ���Լ��������ݲ����� |
���� ��1���Ȼ��������������£�˫��ˮ�ֽ�����������ˮ��
��2�������Թ�A��Bʵ���������ͬ����
��3������Ӱ�컯ѧ��Ӧ���ʵ�������ؽ��
��4���������ʵı�ʾ������ͼ���������������������
��5���ٶ��Է������Ը��ݲ������ݵ������������жϷ�Ӧ�Ŀ�������̽��Ӱ�췴Ӧ���ʵĿ���������ʱͨ����ȡ���Ʊ�����������ʵ�飬ѡ����ʵ����ʣ�
��������������Ӧ�Ŀ��������ռ�һ����������壬ʱ�����Ӧ�죮
��� �⣺��1���Ȼ��������������£�˫��ˮѸ�ٷֽ�����������ˮ����Ӧ����ʽΪ��2H2O2$\frac{\underline{\;�Ȼ���\;}}{\;}$2H2O+O2����
�ʴ�Ϊ��2H2O2$\frac{\underline{\;�Ȼ���\;}}{\;}$2H2O+O2����
��2���ֱ����Թ�A��B�м��� 5mL 5% H2O2��Һ��������1��2 ��1mol/L FeCl3��Һ�����Թ��о����������ݳ��֣�˵����������ֽ��ܷ������Թ�A��B�о����������ݳ���ʱ�����Թ�A����ʢ��5��������ˮ���ձ��У����Թ�B����ʢ��40��������ˮ���ձ��У���֧�Թܲ�ͬ�����Թ�A���¶ȱ��Թ�B���¶ȵͣ�˵���о������¶ȶԷ�Ӧ���ʵ�Ӱ�죬����ʼ�ӵμ�FeCl3��Һ��Ŀ�ļӿ�H2O2�ֽ⣬
�ʴ�Ϊ���о��¶ȶ�H2O2�ֽ����ʵ�Ӱ�죻�ӿ�H2O2�ֽ����ʣ�ʹʵ���������ڹ۲죻
��3��Ӱ�컯ѧ��Ӧ���ʵ����������Ũ�ȡ��¶ȡ������ѹǿ������������ı��������ȡ��֧�Թֱܷ���� 5mL 5%H2O2��Һ�� 5mL10%H2O2��Һ���Թ�A��B�о�δ�����ݲ�����Ϊ�ӿ췴Ӧ���ʣ��ɴ��¶ȡ�������Ӱ��Ƕȿ��ǣ�
�ʴ�Ϊ������֧�Թ�ͬʱ����ʢ����ͬ�¶���ˮ���ձ��У�������֧�Թ���ͬʱ����2��1mol/L FeCl3��Һ���۲�������ݵ����ʣ�
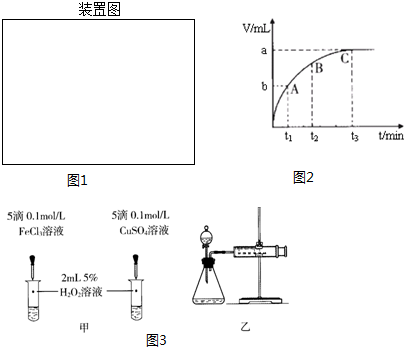
��4����ͼ��������ʾʱ�䣬�������ʾ��������������ʱ��Խ�����ɵ�����Խ�࣬��Ӧ����Խ�죬��������������ΪC���ʴ�Ϊ��C��
��5�������ڶ��Է������Ը��ݲ������ݵ������������жϷ�Ӧ�Ŀ�����Ϊ�Ƚ�Fe3+��Cu2+��H2O2�ֽ�Ĵ�Ч����Ҫ��������������ͬ����FeCl3��Һ�л��������ӣ�CuSO4�к���ͭ���ӣ���������ӿ���Ҳ��Ӱ�췴Ӧ���ʣ��������ţ����Կɽ�FeCl3��ΪFe2��SO4��3��
�ʴ�Ϊ����Һ�����ݲ��������ʣ��ų������ӵĸ��ţ��������ɣ���
��������������Ӧ�Ŀ������Բⶨ�ռ�40mL�����������ʱ�䣬ʱ�����Ӧ�죬
�ʴ�Ϊ���ռ�40mL���������ʱ�䣮
���� ������Ҫ����ѧ����ʵ��̽�������������ݴ������ص�Ϳ��Ʊ�������˼��������⼰��Ʒ������ۺ��ԱȽ�ǿ���ѶȽϴ�


 ��ʦ����ɳ���ʱͬ��ѧ����ϵ�д�
��ʦ����ɳ���ʱͬ��ѧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
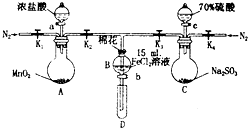 Ϊ��֤������Cl2��Fe3+��SO2��ijС������ͼ��ʾװ�ý���ʵ�飨�г�������A�м���װ�����ԣ��������Ѽ��飩��
Ϊ��֤������Cl2��Fe3+��SO2��ijС������ͼ��ʾװ�ý���ʵ�飨�г�������A�м���װ�����ԣ��������Ѽ��飩��| ���̢���B��Һ�к��е����� | ���̢���B��Һ�к��е����� | |
| �� | ��Fe3+��Fe2+ | ��SO42- |
| �� | ����Fe3+����Fe2+ | ��SO42- |
| �� | ��Fe3+��Fe2+ | ��Fe2+ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ˮ | B�� | ��ˮ | C�� | ���� | D�� | �Ȼ��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | CH4��C4H10��Ϊͬϵ�� | |
| B�� | 23592U��23892U��������ͬ����������ͬһ�ֺ��� | |
| C�� | ���Ǻ���ѿ�Ƕ�����˫�ǣ��һ�Ϊͬ���칹�� | |
| D�� | �������е�̼̼���ǽ��ڵ�����˫��֮��Ķ��صļ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �ڢܢߢ���� | B�� | �ڢܢߢ�� | C�� | �ۢܢߢ� | D�� | �ۢܢݢߢ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
| ʵ����� | �Լ� | ���� |
| I | ����Ag2SO4��Һ | ������ɫ���� |
| II | 0.2mol•L-1CuCl2��Һ | ��Һ���̣������μӲ����ػ�ɫ���� |
| III | 0.1mol•L-1Al2��SO4��3��Һ | ��ʼ�����Ա仯�������μӲ�����ɫ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 1.0 mol•L-1 KNO3��Һ��H+��Fe2+��SCN-��SO42- | |
| B�� | $\frac{{K}_{w}}{c��{H}^{+}��}$=1��10-13mol•L-1����Һ��NH4+��Ca2+��Cl-��NO3- | |
| C�� | pH=1����Һ��Al3+��Ag��NH3��2+��Cl-��SO42- | |
| D�� | ��ˮ�������c��H+��=10-10mol/L����Һ��NH4+��Ca2+��AlO2-��S2- |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com