

���� ij��ҺA�п��ܺ���NH4+��Fe3+��Al3+��Fe2+��CO32-��NO3-��Cl-��SO42-�еļ������ӣ�����Һ�и����ӵ����ʵ���Ũ�Ⱦ�Ϊ0.1mol/L��
����Һ�м�������ģ�NH4��2CO3��Һ�����ɵ���ɫ�����ΪCO2�����ɵİ�ɫ��������CO32-����Һ�е�����������˫ˮ�����ɵģ��������ɵij���Ϊ��ɫ���ʴ�����������ΪAl3+������˵����Һ�в���Fe3+��Fe2+��
����Һ�м��������Ba��OH��2��Һ�����ɵ�������ΪNH3������ǰ�����Ĺ����ģ�NH4��2CO3��Һ������NH4+���ʲ���ȷ��ԭ��Һ�к�NH4+��
ǰ�����Ĺ����ģ�NH4��2CO3��Һ������CO32-�������ɵİ�ɫ������һ����BaCO3��
����Һ���м�ͭ�����ᣬ������������ɣ�˵����Һ�к�NO3-��
���Ϸ�����֪����Һ��һ����CO32-��Fe3+��Fe2+��һ����0.1mol/L H+��0.1mol/LAl3+��0��1mol/LNO3-��������Һ�����Ե����ԣ�����Һ��һ����Cl-��SO42-���ݴ˽��н��1����2����3����
��4�������������ܽ�ǰ笠��������Ƚ�����������ӣ������ﵽ�����ʱ��������û���ܽ⣬֤����Һ�к���笠����ӣ�
�ڸ��������ӵ����ʵ������������������Һ��Ũ�ȣ�
�۸����������������ʵ����������ӡ�̼������ӡ���������ӵ����ʵ������м������ɳ����������ʵ�����
��� �⣺ij��ҺA�п��ܺ���NH4+��Fe3+��Al3+��Fe2+��CO32-��NO3-��Cl-��SO42-�еļ������ӣ�����Һ�и����ӵ����ʵ���Ũ�Ⱦ�Ϊ0.1mol/L��
����Һ�м�������ģ�NH4��2CO3��Һ�����ɵ���ɫ�����ΪCO2�����ɵİ�ɫ��������CO32-����Һ�е�����������˫ˮ�����ɵģ��������ɵij���Ϊ��ɫ���ʴ�����������ΪAl3+������˵����Һ�в���Fe3+��Fe2+��
����Һ�м��������Ba��OH��2��Һ�����ɵ�������ΪNH3������ǰ�����Ĺ����ģ�NH4��2CO3��Һ������NH4+���ʲ���ȷ��ԭ��Һ�к�NH4+��
ǰ�����Ĺ����ģ�NH4��2CO3��Һ������CO32-�������ɵİ�ɫ������һ����BaCO3��
����Һ���м�ͭ�����ᣬ������������ɣ�˵����Һ�к�NO3-��
���Ϸ�����֪����Һ��һ����CO32-��Fe3+��Fe2+��һ����0.1mol/L H+��0.1mol/LAl3+��0��1mol/LNO3-��������Һ�����Ե����ԣ�����Һ��һ����Cl-��SO42-��
��1�����ݷ�����֪����ҺA��һ�������ڵ�����ΪFe3+��Fe2+��CO32-��
�ʴ�Ϊ��Fe3+��Fe2+��CO32-��
��2����ҺA�м��루NH4��2CO3��CO32-��Al3+��ˮ�ⷴӦ��ٽ��Ӷ�����Al��OH��3������CO2���壬
�ʴ�Ϊ��CO32-��Al3+��ˮ�ⷴӦ��ٽ��Ӷ�����Al��OH��3������CO2���壻
��3����ɫ��������һ������BaCO3��BaSO4������
�ʴ�Ϊ��BaCO3��BaSO4 ��
��4���ٵ������ﵽ���ֵʱ���ټ�������������Һ�����û�������ܽ⣬��ʱ笠����������������ӷ�Ӧ��˵����Һ��һ������NH4+��
�ʴ�Ϊ�����У�
��10mLԭ��Һ�к��������ӵ����ʵ���Ϊ��0.1mol/L��0.01L=0.001mol��0.001mol��������ȫ��Ӧ�����������Ƶ����ʵ���Ϊ0.003mol������ͼ���֪����������ȫת������������������������������Һ�����Ϊ15mL��
������������Һ��Ũ��Ϊ��$\frac{0.003mol}{0.015L}$=0.2mol/L��
�ʴ�Ϊ��0.2��
������A��Һ�иļ�10ml0.2mol��L-1Ba��OH��2��Һ�������������������ʵ���Ϊ��0.2mol/L��0.01L=0.002mol��0.002mol����������Һ�к���0.004mol���������Ӻ�0.002mol�����ӣ�
10mLA��Һ�к���笠����ӵ����ʵ���Ϊ0.001mol��������������Ӻ�̼������ӵ����ʵ����ֱ�Ϊ0.001mol������10mLA��Һ�м���0.002mol����������Һ�����ɳ���Ϊ0.001mol����������0.001mol���ᱵ��̼�ᱵ���������ɳ����������ʵ���Ϊ0.002mol��
�ʴ�Ϊ��0.002��
���� ���⿼�������ƶϼ��������ӵļ��鷽������Ŀ�Ѷ��еȣ���ȷ�������ӵ�����Ϊ���ؼ���ע�����ճ������ӵľ��鷽��������������ѧ���ķ������������Ӧ��������



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �������������ᷴӦ��H++OH-�TH2O | |
| B�� | ��С�մ�����θ����ࣺHCO3-+H+�TCO2��+H2O | |
| C�� | �������ᷴӦ��2Fe+6H+�T2Fe3++3H2�� | |
| D�� | CaCO3����ϡ�����У�CO32-+2H+�TCO2��+H2O |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | CH4��g��+2O2��g���TCO2��g��+H2O��l����H=-890kJ | |
| B�� | ��ʾH2S����ȼ���ȵ��Ȼ�ѧ����ʽΪ��2H2S��g��+O2��g���T2S��s��+2H2O��l����H=-136kJ/mol | |
| C�� | 2mol H2ȼ�յ�ˮ��������484 kJ����H2O��g���TH2��g��+1/2O2��g����H=+242 kJ/mol | |
| D�� | 2NO+O2=2NO2 ��H=+116.2kJ/mol |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��ȥMgCl2��Һ��������FeCl3����ѡ��MgO | |
| B�� | Na�������������������NaHCO3 | |
| C�� | ��������ͭ�ڿ����г�ʱ����ã������ֻ���������� | |
| D�� | ��NaOH��Һ����μ�����������FeCl3��Һ�����Ƶ�Fe��OH��3���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| ��� | ��Ӧ�� | ���� |
| �� | Cl2��H2O2 | Cl- |
| �� | Cl2��FeI2 | FeCl2��I2 |
| �� | KClO3��HCl | Cl2��KCl��H2O |
| A�� | �ڢ��鷴Ӧ����������ΪO2 | |
| B�� | �ڢ��鷴Ӧ��Cl2 ��FeI2�����ʵ���֮��С�ڻ����1��1 | |
| C�� | �ڢ��鷴Ӧ������1molCl2ת��6mol���� | |
| D�� | ��������ǿ������˳��ΪClO3-��Cl2��I2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��������CO��CO2��O3�ֱ���1mol O����������������ʵ���֮��Ϊ3��2��1 | |
| B�� | ng Cl2����m��Clԭ�ӣ����ӵ�����NA����ֵ�ɱ�ʾΪ$\frac{35.5m}{n}$ | |
| C�� | ��״���£�11.2L X������ӵ�����Ϊ16g����X�����Ħ��������32 | |
| D�� | 30g CO��22.4L CO2�к��е�̼ԭ����һ����� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
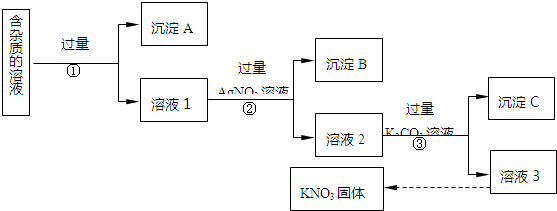
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��Һ��pH���� | B�� | CH3COOH�ĵ���̶����� | ||
| C�� | ��Һ�ĵ���������С | D�� | ��Һ��c ��OH-����С |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com