
| A�� | ������ΪNaA����Һ�п��ܴ��ڣ�c ��OH-��=c��H+��+c��HA�� | |
| B�� | ��c��OH-����c��H+������Һ�в����ܴ���c��Na+����c��OH-����c��A-����c��H+�� | |
| C�� | ��Һ�п��ܴ���c��Na+����c��A-����c��H+����c��OH-�� | |
| D�� | ������ΪNaA��HA����һ������c��A-����c��Na+����c��H+����c��OH-�� |
���� ��Һ��ֻ����Na+��H+��OH-��A-�������ӣ�����Һ�е����ʿ���ΪNaA��NaA��HA��NaA��NaOH�����õ����ˮ������������Ũ�ȵĹ�ϵ��
A��������Һ�������غ�����жϣ�
B����c��OH-����c��H+�����������ʿ���ΪNaA��NaOH������
C�������Ӵ���������Ũ�ȣ���Һ��ɲ��غ㣻
D������ΪNaA��HA��������ĵ���̶Ⱥ��ε�ˮ��̶Ƚ��з�����
��� �⣺A��ˮ�����������������������Ũ����ȣ���HAΪ����ʱ�����������غ��֪��c ��OH-��=c��H+��+c��HA������A����
B����c��OH-����c��H+�������ʿ���ΪNaA��NaOH����NaOH�����ʵ����ϴ�ʱ���Դ��ڣ�c��Na+����c��OH-����c��A-����c��H+������B����
C�������Ӵ���������Ũ�ȣ���Һ��ɲ��غ㣬���c��Na+����c��A-������Ӧ�����㣺c��OH-����c��H+������C����
D��������ΪNaA��HA�����ߵ����ʵ���ʱ�����������ε�ˮ�����c��A-����c��Na+����c��H+����c��OH-���������ߵ����ʵ������ε�ˮ�������ĵ��룬�����c��Na+����c��A-����c��OH-����c��H+������D����
��ѡA��
���� ���⿼������Ũ�ȴ�С�ıȽϣ���Ŀ�Ѷ��еȣ�ע�������ж���Һ������Ũ�ȴ�С�ķ�������ȷ��Һ�е����ʼ����롢ˮ��ij̶��ǽ����Ĺؼ�����ѧ�����õ���غ����������



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | NaCl��Һ | B�� | Fe��OH��3���� | C�� | Ũ���� | D�� | �ཬ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 3��2��1��4 | B�� | 1��1��1��1 | C�� | 1��2��1��2 | D�� | 2��2��1��1 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
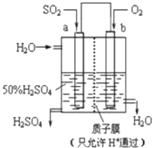
| A�� | a������b���� | |
| B�� | ����������H+��a�缫�����˶� | |
| C�� | ���Ӵ�b����a���ƶ� | |
| D�� | ������ӦʽΪ��2H2+SO2-2e-=SO42-+4H+ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ������Һ���� | B�� | CH3COONa��Һ���� | ||
| C�� | ��ˮ�м�������NH4Cl | D�� | Na2CO3��Һ�м���BaCl2���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ƶ���
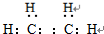 ��B�Ľṹ��ʽ
��B�Ľṹ��ʽ ��
�� ��
���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com